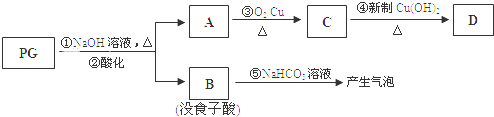
��Ŀ����
10�������к��зḻ�ĵ⣬Ϊ�˴Ӻ�������ȡ�⣬ij�о���ѧϰС����Ʋ���������ͼ��ʾʵ�飺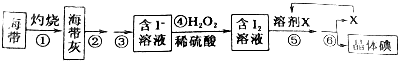
����д�������⣺
��1��ʵ�������պ���Ӧ�����������������ƣ��ڽ��У�
��2������ܷ�Ӧ�����ӷ���ʽ��2I-+H2O2+2H+=I2+2H2O��
��3��������У�Ӧѡ�õ�һ���ܼ�X�����DZ������Ȼ�̼�����Լ����ƣ���
��4�����������е�ʵ���������ͼ�е�ADC��������˳��ѡ����ţ���

��5�����һ����ʵ�飬������ȡ������Һ���Ƿ��е��ʵ�ȡ��������Һ���Թ��У����������ĵ��ۣ������Һ��������˵����ȡ������Һ�л����е��ʵ⣬�������ⵥ�ʣ���
���� ��1������ʵ���������������������õ���ʵ��������
��2��H2O2���н�ǿ�������ԣ������������¿����������ӣ�
��3����ȡ����ѡ��Ҫ���㣺��������ȡ���е��ܽ�ȴ���ԭ�ܼ���ԭ�ܼ�����ȡ���������ܣ�
��4����������ͼ�еIJ����ֱ�Ϊ���ա��ܽ⡢���ˡ���ȡ��������������
��5���������������
��� �⣺��1�����չ�������һ��ʹ�ã��ɣ��������ʴ�Ϊ��������
��3�������������������¿ɱ�H2O2�����ɵ��ʵ⣬H2O2����ԭΪˮ����Ӧ�����ӷ���ʽΪ2I-+H2O2+2H+=I2+2H2O��
�ʴ�Ϊ��2I-+H2O2+2H+=I2+2H2O��
��3���ڢݲ��IJ�������ȡ����Ҫ������ȡ��������ʹ�õ���ȡ��Ҫ���㣺��������ȡ���е��ܽ�ȴ���ԭ�ܼ���ԭ�ܼ�����ȡ���������ܣ��ʳ��ñ������Ȼ�̼������ȡ�����ʴ�Ϊ���������Ȼ�̼��
��4����������ͼ��֪���Ƚ��������в��������գ�Ȼ�����ú����ҽ��в������ܽ�Ͳ����۹��˼�ѡ��A��Ȼ�������õ���Һ�м���˫��ˮ��ϡ��������I-ΪI2���������ܣ�Ϊ�˽����ɵĵⵥ����ȡ������Ҫ���в�������ȡ��Һ������D��Ȼ�����õĵ���л���Һ���в�����������C���Ӷ���ȡ���ʵ⣬�ʴ�Ϊ��ADC��
��5�����ڵ��������������Ӧȡ��������Һ���Թ��У����������ĵ��ۣ������Һ��������˵����ȡ������Һ�л����е��ʵ⣬�ʴ�Ϊ��ȡ��������Һ���Թ��У����������ĵ��ۣ������Һ��������˵����ȡ������Һ�л����е��ʵ⣬�������ⵥ�ʣ�
���� ������Ҫ����Ӻ�������ȡ���ʵ�飬��ȷ���ʵķ��뷽�����ⵥ�ʵ������ǽ��Ĺؼ�����Ŀ�Ѷ��еȣ�

 ���100�ֵ�Ԫ�Ż�������ϵ�д�
���100�ֵ�Ԫ�Ż�������ϵ�д�| A�� | Ca2+��CO32-��NO3-��K+ | B�� | Cu2+��K+��SO42-��NO3- | ||
| C�� | Na+��NH4+��SO42-��Cl- | D�� | Ag+��Al3+��OH-��Cl- |
| A�� | H3PO4 | B�� | NaOH | C�� | MgO | D�� | KNO3 |
| A�� | 2HgO$\frac{\underline{\;\;��\;\;}}{\;}$ 2Hg+O2 | B�� | Fe3O4+4CO$\frac{\underline{\;����\;}}{\;}$ 3Fe+4CO2 | ||
| C�� | Fe+CuSO4=Cu+FeSO4 | D�� | 2NaCl�����ڣ�$\frac{\underline{\;����\;}}{\;}$ 2Na+Cl2�� |

 �����¶�����DZͧ���õ�������ȼ�ϵ��������װ�ã���������������������������ȼ�ϵ������1.8Lˮ����ʱ�������ת�Ƶĵ�����Ϊ200NA��
�����¶�����DZͧ���õ�������ȼ�ϵ��������װ�ã���������������������������ȼ�ϵ������1.8Lˮ����ʱ�������ת�Ƶĵ�����Ϊ200NA��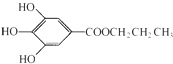 ���ǰ�ɫ��ĩ��������ˮ�����������͵���֬���dz��õ�ʳ���Ϳ���������
���ǰ�ɫ��ĩ��������ˮ�����������͵���֬���dz��õ�ʳ���Ϳ���������