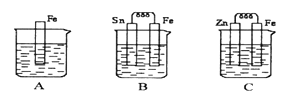
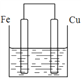
��Ŀ����
����Ŀ����t��Cʱ��AgBr��ˮ�еij����ܽ�ƽ��������ͼ��ʾ����֪t��CʱAgCl��Ksp=4��10-10������˵������ȷ����( )
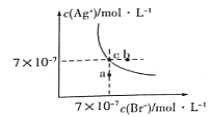
A��ͼ��a���Ӧ����AgBr�IJ�������Һ
B����t ��Cʱ��AgBr��KspΪ 4.9��10-13
C����AgBr������Һ�м���NaBr���壬��ʹ��Һ��c�㵽b��
D����t ��Cʱ��AgCl(s)+Br-(aq)![]() AgBr(s)+C1- (aq)��ƽ�ⳣ��K��816
AgBr(s)+C1- (aq)��ƽ�ⳣ��K��816
���𰸡�C
��������
���������A������ͼ���֪����a��ʱQc=c(Ag+)c(Br-)��Ksp������a��ΪAgBr�IJ�������Һ����A��ȷ��B�����ͼ��c���c(Ag+)��c(Br-)��֪�����¶���AgBr��Ksp=7��10-7��7��10-7=4.9��10-13����B��ȷ��C����AgBr������Һ�м���NaBr�����c(Br-)�����ܽ�ƽ�������ƶ���c(Ag+)��С����C����D����ӦAgCl(s)+Br-(aq)AgBr(s)+Cl-(aq)��ƽ�ⳣ��Ϊ��K=![]() =
=![]() ��816����D��ȷ����ѡC��
��816����D��ȷ����ѡC��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ����HNO3��HF�Ļ��ᴦ��ij������������ϴ��Һ�к���Fe3+��Ni2+��NO3-��F-��Cr2O72-�ȡ���ͼ���ۺ����ø���ϴ��Һ�Ĺ������̣�
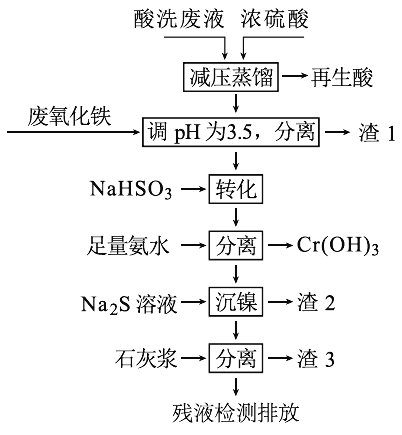
��֪��
�������ӿ�ʼ�����ͳ�����ȫʱ��pH��
Fe3+ | Ni2+ | Cr3+ | |
��ʼ���� | 1.5 | 6.7 | 4.0 |
������ȫ | 3.4 | 9.5 | 6.9 |
��Ni2+��������ˮ�ķ�ӦΪ��Ni2+��6NH3 ![]() [Ni(NH3)6]2+
[Ni(NH3)6]2+
��1���������к���HNO3,��ȡ��ѹ�����Ŀ��Ϊ____________��
��2������������Ҫ�ɷ�Ϊ��____________��
��3����д����ת����ʱNaHSO3��Cr2O72-������Ӧ���������뻹ԭ�������ʵ���֮�ȣ�________��
��4����֪[Ni(NH3)6]2+Ϊ�ѵ����������ӣ������������ӷ���ʽΪ��____________��
��5������3����Ҫ�ɷ�ΪCaSO4��Ca(OH)2��_____��
��6������⣬���IJ�Һ��c(Ca2+)��0.001 molL-1�����Һ��F��Ũ��Ϊ____mgL-1��______������ϡ������ϡ����ŷű�[��֪Ksp(CaF2)=4��10-11�������ŷű�Ҫ�������Ũ��С��10 mgL-1]��