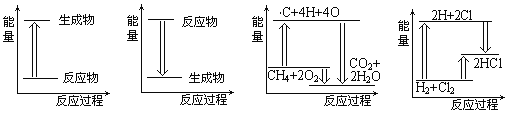
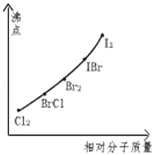
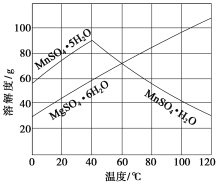
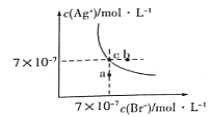
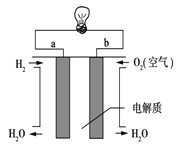
��Ŀ����
����Ŀ��ijˮ��Һ�����ܺ��������������������֣�K����NH4+��Cl����Mg2����Ba2����CO32����SO42������ȡ����100 mL��Һ��������ʵ�飺
�ٵ�һ�ݼ�����NaOH��Һ���Ⱥ��ռ�������0.05 mol��
�ڵڶ��ݼ�����BaCl2��Һ�ø������4.3 g������������ϴ�ӡ������������Ϊ2.33 g������˵����ȷ����
A. K��һ������
B. ��Һ��c(Cl��)����Ϊ0.2 mol/L
C. Cl�����ܴ���
D. Ba2��һ�������ڣ�Mg2�����ܴ���
���𰸡�B
������������һ�ݼ�����NaOH��Һ���Ⱥ��ռ�������0.05 mol��˵����NH4+���������ʵ���Ϊ0.05mol�����ڶ��ݼ�����BaCl2��Һ�ø������4.3g������������ϴ�ӡ������������Ϊ2.33 g��˵������BaSO42.33g�������ʵ���Ϊ0.01mol��BaCO3������Ϊ4.3g-2.33g=1.97g�����ʵ���Ϊ0.01mol����֪��Һ�к���CO32-��SO42-��ͬʱ�ų���Һ�к���Mg2����Ba2+��������Һ�ǵ����Եģ���Һ�п϶�����Cl-�������ʵ�������Ϊn(NH4+)��1-n(CO32-)��2-n(SO42-)��2=0.05mol��1-0.01mol��2-0.01mol��2=0.01mol��A. �ݷ�����֪��Һ�в�һ������K������A����B. ��Һ��c(Cl��)����Ϊ![]() =0.1mol/L������Һ�в�����K������c(Cl��)����Ϊ0.2 mol/L����B��ȷ��C. �з�����֪Cl��һ�����ڣ���C����D. �з�����֪��Mg2����Ba2��һ�������ڣ���D����ΪB��
=0.1mol/L������Һ�в�����K������c(Cl��)����Ϊ0.2 mol/L����B��ȷ��C. �з�����֪Cl��һ�����ڣ���C����D. �з�����֪��Mg2����Ba2��һ�������ڣ���D����ΪB��