��Ŀ����
�£�N2H4���ֳ��������㷺���ڻ���ƽ������л��ϳɼ�ȼ�ϵ�أ�NO2�Ķ�����N2O4���ǻ���г������������Իش���������
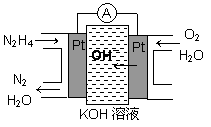
��1����ȼ�ϵ��ԭ����ͼ��ʾ����ߵ缫�Ϸ����ĵ缫��ӦʽΪ_________��
��2���������N2O4��������������ȼ�ϣ���֪��
N2(g)��2O2(g)��2NO2(g) ��H ����67.7kJ��mol-1
N2H4(g)��O2(g)��N2(g)��2H2O(g) ��H ����534.0kJ��mol-1
2NO2(g)![]() N2O4(g) ��H ����52.7kJ��mol-1
N2O4(g) ��H ����52.7kJ��mol-1
��д����̬������̬������������ȼ�����ɵ�������̬ˮ���Ȼ�ѧ��________��
��3�������Ĺ�ҵ�������ð��ʹ�������Ϊԭ�ϻ�ã�д����Ӧ�����ӷ���ʽΪ_ __��
��4����ͼ��ʾ��A�������Ȳ����Ƴɵ����������ܱ�������B��һ�ͻ�ѧ��ʴ�����ڴ��ȵ�����ɱ�������ң�����ı���������嵯���ı���Բ��ƣ����ر�K2������1 mol NO2ͨ��K1��K3�ֱ����A��B�У���Ӧ��ʼʱA��B�������ͬ��Ϊa L��
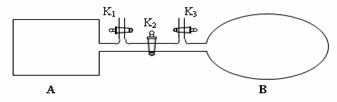
��B�п�ͨ��________________�жϿ��淴Ӧ2NO2![]() N2O4�Ѿ��ﵽƽ�⡣
N2O4�Ѿ��ﵽƽ�⡣
������K2��ƽ���B�������������0.4a L�����K2֮ǰ������B���Ϊ______L��
��������A�е���ƽ������ʱ��t s���ﵽƽ���������ѹǿΪ��ʼѹǿ��0.8������ ƽ����ѧ��Ӧ����v(NO2)����____________________(�ú�a��t�Ĵ���ʽ��ʾ)��
����ƽ�����A�������ٳ���0.5mol NO2�������µ���ƽ���ƽ��������NO2���������_______________���� �������С�����䡱����
��1��N2H4��4e����4OH����N2��4H2O
��2��2N2H4(g)��N2O4(g)��3N2(g)��4H2O(g) ��H����947.6 kJ�� mol��1
(3) 2NH3+CIO-=N2H4+Cl-+H2O
��4��
������B��������ټ�С��������ɫ���ٱ仯�����������𰸸��֣�
��0.7L���� ![]() mol��L-1��s-1�ܱ�С
mol��L-1��s-1�ܱ�С

 �£�N2H4���ֳ���������һ�ֿ�ȼ��Һ�壬�������������ﷴӦ�������ɵ�����ˮ���������ȼ�ϣ�
�£�N2H4���ֳ���������һ�ֿ�ȼ��Һ�壬�������������ﷴӦ�������ɵ�����ˮ���������ȼ�ϣ�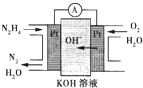 �£�N2H4���ֳ��������㷺���ڻ���ƽ������л��ϳɼ�ȼ�ϵ�أ�NO2�Ķ�����N2O4���ǻ���г��õ�����������ش��������⣺
�£�N2H4���ֳ��������㷺���ڻ���ƽ������л��ϳɼ�ȼ�ϵ�أ�NO2�Ķ�����N2O4���ǻ���г��õ�����������ش��������⣺
 �£�N2H4���ֳ���������һ�ֿ�ȼ��Һ�壬�������ȼ�ϣ�
�£�N2H4���ֳ���������һ�ֿ�ȼ��Һ�壬�������ȼ�ϣ� �£�N2H4���ֳ����������������ȼ�ϣ���һ����ȼ�ϵ���еĵ������Һ��20%��30%��KOH��Һ����ȼ�ϵ�ؿ���Ϊ��ͼװ���еĵ�Դ�������жϴ�����ǣ�������
�£�N2H4���ֳ����������������ȼ�ϣ���һ����ȼ�ϵ���еĵ������Һ��20%��30%��KOH��Һ����ȼ�ϵ�ؿ���Ϊ��ͼװ���еĵ�Դ�������жϴ�����ǣ�������