��Ŀ����
2012��ʼ������������������Ű���纪�������У�ȼú������β������ɿ�����Ⱦ��ԭ��֮һ��
��1������β����������Ҫԭ��Ϊ��2NO(g) + 2CO(g) 2CO2(g)+ N2(g)����H��0
2CO2(g)+ N2(g)����H��0
�ٸ÷�Ӧƽ�ⳣ������ʽ
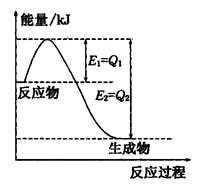
�����÷�Ӧ�ھ��ȡ����ݵ��ܱ���ϵ�н��У�����ʾ��ͼ��ȷ����˵����Ӧ�ڽ��е�t1ʱ�̴ﵽƽ��״̬���� ������ţ���

��2��ֱ���ŷ�úȼ�ղ������������������صĻ������⡣
úȼ�ղ����������������������CH4����ԭNOX�������������������Ⱦ��
��֪���� CH4(g)+2NO2(g)��N2(g)��CO2(g)+2H2O(g)����H����867 kJ/mol
�� 2NO2(g) N2O4(g) ��H����56.9 kJ/mol
N2O4(g) ��H����56.9 kJ/mol
�� H2O(g) �� H2O(l) ��H �� ��44.0 kJ��mol
д��CH4����ԭN2O4(g)����N2��H2O(l)���Ȼ�ѧ����ʽ�� ��
��3������ȼ�ϵ�ؿ����������������ʡ���ͼ�����ü���ȼ�ϵ�ص��100mL1mol/Lʳ��ˮ,���һ��ʱ����ռ�����״���µ�����2.24L���������Һ������䣩.
�ټ���ȼ�ϵ�صĸ�����Ӧʽ�� ��
�ڵ�����Һ��pH= (��������������������Һ��Ӧ)
�������������������ڱ�״������ L
��1������β����������Ҫԭ��Ϊ��2NO(g) + 2CO(g)
 2CO2(g)+ N2(g)����H��0
2CO2(g)+ N2(g)����H��0�ٸ÷�Ӧƽ�ⳣ������ʽ
�����÷�Ӧ�ھ��ȡ����ݵ��ܱ���ϵ�н��У�����ʾ��ͼ��ȷ����˵����Ӧ�ڽ��е�t1ʱ�̴ﵽƽ��״̬���� ������ţ���

��2��ֱ���ŷ�úȼ�ղ������������������صĻ������⡣
úȼ�ղ����������������������CH4����ԭNOX�������������������Ⱦ��
��֪���� CH4(g)+2NO2(g)��N2(g)��CO2(g)+2H2O(g)����H����867 kJ/mol
�� 2NO2(g)
 N2O4(g) ��H����56.9 kJ/mol
N2O4(g) ��H����56.9 kJ/mol �� H2O(g) �� H2O(l) ��H �� ��44.0 kJ��mol
д��CH4����ԭN2O4(g)����N2��H2O(l)���Ȼ�ѧ����ʽ�� ��
��3������ȼ�ϵ�ؿ����������������ʡ���ͼ�����ü���ȼ�ϵ�ص��100mL1mol/Lʳ��ˮ,���һ��ʱ����ռ�����״���µ�����2.24L���������Һ������䣩.
�ټ���ȼ�ϵ�صĸ�����Ӧʽ�� ��
�ڵ�����Һ��pH= (��������������������Һ��Ӧ)
�������������������ڱ�״������ L

��1����[CO2]2[ N2]/[ NO]2[CO]2�� bd
��2��CH4(g)+N2O4(g) =N2(g) +2H2O(g) + CO2(g) ��H= ��898.1kJ/mol
��3����CH4 ��8e�� + 2H2O =CO2 + 8H+ ��14 �� 1.68L
��2��CH4(g)+N2O4(g) =N2(g) +2H2O(g) + CO2(g) ��H= ��898.1kJ/mol
��3����CH4 ��8e�� + 2H2O =CO2 + 8H+ ��14 �� 1.68L
�����������1���ٸ��ݻ�ѧƽ�ⳣ������д���ɣ�������ƽ��Ũ��ϵ�����ݵĻ��ȷ�Ӧ��ƽ��Ũ��ϵ�����ݵĻ���д��[CO2]2[ N2]/[ NO]2[CO]2����a�����淴Ӧ��ƽ�����������������Ҳ��ٱ仯����ͼ��֪��t1ʱ��V������淴Ӧ�������ʷ����仯��δ����ƽ�⣬����b���÷�Ӧ����ӦΪ���ȷ�Ӧ���淴Ӧ�����¶����ߣ���ѧƽ�ⳣ����С������ƽ����¶�Ϊ��ֵ��ƽ�ⳣ�����䣬ͼ����ʵ�ʷ��ϣ���ȷ��c��t1ʱ�̺������̼��NO�����ʵ��������仯��t1ʱ��δ����ƽ��״̬������d�����ŷ�Ӧ�Ľ���NO������������С��t1ʱ��NO�������������䣬����ƽ��״̬����ȷ����bd��
��2����֪���� CH4(g)+2NO2(g)��N2(g)��CO2(g)+2H2O(g)����H����867 kJ/mol �� 2NO2(g)
 N2O4(g) ��H����56.9 kJ/mol �� H2O(g) �� H2O(l) ��H �� ��44.0 kJ��mol�����ݸ�˹���ɣ���-��+���2��CH4(g)+N2O4(g) =N2(g) +2H2O(g) + CO2(g) ��H= ��898.1kJ/mol��
N2O4(g) ��H����56.9 kJ/mol �� H2O(g) �� H2O(l) ��H �� ��44.0 kJ��mol�����ݸ�˹���ɣ���-��+���2��CH4(g)+N2O4(g) =N2(g) +2H2O(g) + CO2(g) ��H= ��898.1kJ/mol����3���ټ���ȼ�ϵ���м����ڸ�������������Ӧ��������������CO2-��H2O�������缫��ӦʽΪCH4 ��8e�� + 2H2O =CO2 + 8H+ ���ڸ�������֪��n(NaCl)=0.1mol�õ�����Ϊ�ȵ�ⱥ��ʳ��ˮ������ˮ�����ݵ�ⷽ��ʽ2Cl- + 2H2O
 H2�� + Cl2�� + 2OH-���������ݼ����n(OH-)=0.1mol��Ũ��Ϊ1mol/L��������Һ��pH=14����n(NaCl)=0.1mol�����������������ʵ���Ϊ0.05mol����������õ�����Ϊ�ȵ�ⱥ��ʳ��ˮ������ˮ���缫��ӦʽΪ2H+ + 2e-=H2�� 2Cl- - 2e-=Cl2�� 4OH- - 4e-=O2�� + 2H2O���������������������ʵ���Ϊ0.1mol�����ݵ����غ�ɵã�2n��H2��=2n��Cl-��+4n��O2���������ݼ����n��O2��=0.025mol,��������������0.075mol,���Ϊ1.68L��
H2�� + Cl2�� + 2OH-���������ݼ����n(OH-)=0.1mol��Ũ��Ϊ1mol/L��������Һ��pH=14����n(NaCl)=0.1mol�����������������ʵ���Ϊ0.05mol����������õ�����Ϊ�ȵ�ⱥ��ʳ��ˮ������ˮ���缫��ӦʽΪ2H+ + 2e-=H2�� 2Cl- - 2e-=Cl2�� 4OH- - 4e-=O2�� + 2H2O���������������������ʵ���Ϊ0.1mol�����ݵ����غ�ɵã�2n��H2��=2n��Cl-��+4n��O2���������ݼ����n��O2��=0.025mol,��������������0.075mol,���Ϊ1.68L��
��ϰ��ϵ�д�
 ����С״Ԫ��������������ϵ�д�
����С״Ԫ��������������ϵ�д� ����������ϵ�д�
����������ϵ�д�
�����Ŀ
 CH3OH ( g ) ��H��-90.8 kJ��mol��1 ��һ�ݻ��ɱ���ܱ������г���10 mol CO ��20 molH2��CO ��ƽ��ת�������¶ȣ�T����ѹǿ��P���ı仯��ͼ��ʾ�����ﵽƽ��״̬A ʱ�����������Ϊ20 L��
CH3OH ( g ) ��H��-90.8 kJ��mol��1 ��һ�ݻ��ɱ���ܱ������г���10 mol CO ��20 molH2��CO ��ƽ��ת�������¶ȣ�T����ѹǿ��P���ı仯��ͼ��ʾ�����ﵽƽ��״̬A ʱ�����������Ϊ20 L��

 CO��g��+H2O��g�� ��H=" +" 41.3 kJ��mol��1�������
CO��g��+H2O��g�� ��H=" +" 41.3 kJ��mol��1�������
 CH3OH(g)
CH3OH(g)




 CH3OH(g)��H2O(g) ��H����49.0kJ/mol
CH3OH(g)��H2O(g) ��H����49.0kJ/mol
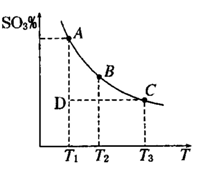
 2SO3��g���������ϵ��SO3 �İٷֺ������¶ȵĹ�ϵ����ͼ��ʾ���������κ�һ�㶼��ʾƽ��״̬��������ͼʾ�ش��������⣬
2SO3��g���������ϵ��SO3 �İٷֺ������¶ȵĹ�ϵ����ͼ��ʾ���������κ�һ�㶼��ʾƽ��״̬��������ͼʾ�ش��������⣬
 ���>����<����=����
���>����<����=����