��Ŀ����
���ǵ����Ϻ����ḻ��һ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���á���ش��������⣺
��1����ͼ��N2��H2��Ӧ����2 mol NH3������������
��ʾ��ͼ��д������NH3���Ȼ�ѧ����ʽ��
_____________________________________________
___________________________��
��2������̬��̬ԭ���γ�1 mol��ѧ���ͷŵ���������м��ܡ��ӻ�ѧ���ĽǶȷ�������
ѧ��Ӧ�Ĺ��̾��Ƿ�Ӧ��Ļ�ѧ�����ƻ���������Ļ�ѧ�����γɹ��̡��ڻ�ѧ��Ӧ��
���У���ѧ����Ҫ�����������γɻ�ѧ���ֻ��ͷ�������
��֪��ӦN2(g)��3H2(g)??2NH3(g)����H��a kJ��mol��1��
�Ը��ݱ������м������ݹ���a����ֵ��________��
��1����ͼ��N2��H2��Ӧ����2 mol NH3������������
��ʾ��ͼ��д������NH3���Ȼ�ѧ����ʽ��
_____________________________________________
___________________________��
��2������̬��̬ԭ���γ�1 mol��ѧ���ͷŵ���������м��ܡ��ӻ�ѧ���ĽǶȷ�������
ѧ��Ӧ�Ĺ��̾��Ƿ�Ӧ��Ļ�ѧ�����ƻ���������Ļ�ѧ�����γɹ��̡��ڻ�ѧ��Ӧ��
���У���ѧ����Ҫ�����������γɻ�ѧ���ֻ��ͷ�������
��֪��ӦN2(g)��3H2(g)??2NH3(g)����H��a kJ��mol��1��
�Ը��ݱ������м������ݹ���a����ֵ��________��
| ��ѧ�� | H��H | N��H | N��N |
| ����kJ��mol��1 | 436 | 391 | 945 |
��1��N2(g)��3H2(g)??2NH3(g) ��H����92.2 kJ��mol��1����2����93
�����������1������������ϵͼ���÷�Ӧ�Ħ�H��E1��E2��(335��427.2)kJ��mol��1����92.2 kJ��mol��1����2����H�����յ��������ͷŵ�������(945��3��436��6��391)kJ��mol��1����93 kJ��mol��1��

��ϰ��ϵ�д�
 Сѧ���AB��ϵ�д�
Сѧ���AB��ϵ�д�
�����Ŀ
 2 Na2CO3��l��+ C(s,���ʯ) ��H=��1080��9kJ/mol
2 Na2CO3��l��+ C(s,���ʯ) ��H=��1080��9kJ/mol

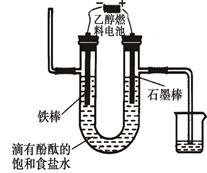
 2CO2(g)+ N2(g)����H��0
2CO2(g)+ N2(g)����H��0
 N2O4(g) ��H����56.9 kJ/mol
N2O4(g) ��H����56.9 kJ/mol 
 O2��g��= ZnO (s) ��H=" -" Q1 kJ? mol-1 ��
O2��g��= ZnO (s) ��H=" -" Q1 kJ? mol-1 �� H= ?241��8kJ/mol
H= ?241��8kJ/mol  2SO3(g)��
2SO3(g)�� 2NO(g)
2NO(g)  2SO3(g)��д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽ��
2SO3(g)��д���÷�Ӧ�Ļ�ѧƽ�ⳣ������ʽ�� 
 SO3(g)+NO(g)�������Ϊ1��2��NO2��SO2���������ܱ������з���������Ӧ��������˵����Ӧ�ﵽƽ��״̬���� ��
SO3(g)+NO(g)�������Ϊ1��2��NO2��SO2���������ܱ������з���������Ӧ��������˵����Ӧ�ﵽƽ��״̬���� ��


 CH3OH��g������H=-90.8kJ��mol��1
CH3OH��g������H=-90.8kJ��mol��1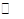 CH3OCH3��g��+H2O(g)ij�¶��µ�ƽ�ⳣ��Ϊ400��
CH3OCH3��g��+H2O(g)ij�¶��µ�ƽ�ⳣ��Ϊ400��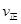 _______
_______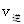 ���>������<����=������
���>������<����=������