��Ŀ����
����ú��ƶ�͡����������ҹ���Դ��չ���ٵ���״��������Դ��������ţ���չ��ú���������ҹ���Դ�ṹ�ĵ���������Ҫ���塣��ͼ��ú������ҵ��֮һ��

���ྻú�������о����������൱�ձ飬������Աͨ�������ú������¯�н�����������ˮ�����ķ���������������ֵ�ܸߵ�ú̿�ϳ���������Ҫ�ɷ���CO��H2��CO��H2����Ϊ��Դ�ͻ���ԭ�ϣ�Ӧ��ʮ�ֹ㷺��
��1����֪��C(s)+O2(g)=CO2(g) ��H1����393.5 kJ��mol�C1 ��
C(s)+H2O(g)=CO(g)+H2(g) ��H2����131.3 kJ��mol�C1 ��
��ӦCO(g)+H2(g) +O2(g)= H2O(g)+CO2(g)����H= _________kJ��mol�C1���ڱ�״���£�33.6 L��ú̿�ϳ���(��ȫ��ΪCO��H2)��������ȫ��Ӧ����CO2��H2O����Ӧ��ת��______mole����
��2����һ���ݵ��ܱ������У���CO��H2�ϳɼ״���CO(g)+2H2(g) CH3OH(g)
CH3OH(g)
������������˵��������Ӧ�Ѵﵽƽ��״̬����_______
a����ϵѹǿ���ֲ���
b���ܱ�������CO��H2��CH3OH(g)3�����干��
c��CH3OH��H2���ʵ���֮��Ϊ1:2
d��ÿ����1 mol CO��ͬʱ����2molH2
��CO��ƽ��ת����(��)���¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��

A��B�����ƽ�ⳣ��_____(�ǰ�ߡ��������ߡ���һ����)�ﵽA��C�����ƽ��״̬�����ʱ��tA tC(����ڡ�����С�ڡ����ڡ�)��
�ڲ��ı䷴Ӧ������������£�Ϊ���CO��ת���ʿɲ�ȡ�Ĵ�ʩ��_____________(������㼴��)��
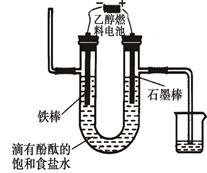
��3�������¶�650���������ȼ�ϵ�أ�����ú̿��(CO��H2)������ȼ����������CO2�Ļ������Ϊ����ȼ������һ��������Li2CO3��Na2CO3���۵�����������ʣ��Խ�����(ȼ�ϼ�)Ϊ�����Ƴɵġ������ĵ缫��ӦʽΪ��CO + H2��4e�� + 2CO32��= 3CO2+H2O����õ�ص�������ӦʽΪ____________��

���ྻú�������о����������൱�ձ飬������Աͨ�������ú������¯�н�����������ˮ�����ķ���������������ֵ�ܸߵ�ú̿�ϳ���������Ҫ�ɷ���CO��H2��CO��H2����Ϊ��Դ�ͻ���ԭ�ϣ�Ӧ��ʮ�ֹ㷺��
��1����֪��C(s)+O2(g)=CO2(g) ��H1����393.5 kJ��mol�C1 ��
C(s)+H2O(g)=CO(g)+H2(g) ��H2����131.3 kJ��mol�C1 ��
��ӦCO(g)+H2(g) +O2(g)= H2O(g)+CO2(g)����H= _________kJ��mol�C1���ڱ�״���£�33.6 L��ú̿�ϳ���(��ȫ��ΪCO��H2)��������ȫ��Ӧ����CO2��H2O����Ӧ��ת��______mole����
��2����һ���ݵ��ܱ������У���CO��H2�ϳɼ״���CO(g)+2H2(g)
 CH3OH(g)
CH3OH(g)������������˵��������Ӧ�Ѵﵽƽ��״̬����_______
a����ϵѹǿ���ֲ���
b���ܱ�������CO��H2��CH3OH(g)3�����干��
c��CH3OH��H2���ʵ���֮��Ϊ1:2
d��ÿ����1 mol CO��ͬʱ����2molH2
��CO��ƽ��ת����(��)���¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��

A��B�����ƽ�ⳣ��_____(�ǰ�ߡ��������ߡ���һ����)�ﵽA��C�����ƽ��״̬�����ʱ��tA tC(����ڡ�����С�ڡ����ڡ�)��
�ڲ��ı䷴Ӧ������������£�Ϊ���CO��ת���ʿɲ�ȡ�Ĵ�ʩ��_____________(������㼴��)��
��3�������¶�650���������ȼ�ϵ�أ�����ú̿��(CO��H2)������ȼ����������CO2�Ļ������Ϊ����ȼ������һ��������Li2CO3��Na2CO3���۵�����������ʣ��Խ�����(ȼ�ϼ�)Ϊ�����Ƴɵġ������ĵ缫��ӦʽΪ��CO + H2��4e�� + 2CO32��= 3CO2+H2O����õ�ص�������ӦʽΪ____________��
��1�� ��524.8 (2��) 3 (2��)
��2�� ��a d (2��)
��һ�� (2��) ���� (2��) ���¡���ѹ�����״��ӻ����ϵ�з������ (2��)
��3�� O2 + 4e�C + 2CO2 = 2CO32�C (2��)
��2�� ��a d (2��)
��һ�� (2��) ���� (2��) ���¡���ѹ�����״��ӻ����ϵ�з������ (2��)
��3�� O2 + 4e�C + 2CO2 = 2CO32�C (2��)
�����������1�����ݸ�˹���ɣ��������Ȼ�ѧ����ʽ��������٣��ڵã�CO(g)+H2(g) +O2(g)= H2O(g)+CO2(g)����H=��H1����H2����393.5 kJ��mol�C1��131.3 kJ��mol�C1����524.8kJ��mol�C1��CO��CO2ת��2mole�C��H2��H2Oת��2mole�C����1.5molCO��H2�Ļ��������O2��ȫ��Ӧ����CO2��H2Oʱ��ת��3mol���ӡ���2����a������ӦΪ�����������С�ķ�Ӧ����ѹǿ����ʱ��˵��������Ũ�Ȳ��ٱ仯����Ӧ�ﵽƽ��״̬��b��CO��H2��CH3OH(g)3�����干��ʱ������ȷ��Ũ���Ƿ�ᷢ���仯��������ȷ���Ƿ���һ���ﵽƽ��״̬��c��CH3OH��H2���ʵ���֮��Ϊ1:2������ȷ����Ũ�Ȳ��䣬��ˣ�����ȷ���Ƿ���һ���ﵽƽ��״̬����d��������1 mol CO��ͬʱ����2molH2ʱ��˵�����淴Ӧ������ȣ�����Ӧ�ﵽƽ��״̬����A��B������¶���ͬ������ƽ�ⳣ����ȣ���ͼ����A��C�����ת������ȣ���C����¶ȸߣ���Ӧ���ʴ���ƽ���ʱ��̡���ͼ�����¶Ƚ���CO��ת�������÷�ӦΪ�����������С�ķ�Ӧ����ѹ��ʹƽ��������Ӧ�����ƶ���CO��ת��������c(CH3OH)��С��ƽ��Ҳ��������Ӧ�����ƶ���CO��ת��������3��ȼ�յ�ص�����ΪO2�õ��ӣ����ǵ�����ܷ�ӦΪH2��CO��O2��Ӧ����CO2��H2O����������CO32�C���뷴Ӧ��������Ӧ����CO32�C���ɣ���������ӦʽΪ��O2 + 4e�C + 2CO2 = 2CO32�C��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ

 2 Na2CO3��l��+ C(s,���ʯ) ��H=��1080��9kJ/mol
2 Na2CO3��l��+ C(s,���ʯ) ��H=��1080��9kJ/mol

 CH3OH(g)��ͼ1��ʾ��Ӧ�е������仯��ͼ2��ʾһ���¶��£������Ϊ1L���ܱ������м���2mol H2��һ������CO��CO��CH3OH(g)��Ũ����ʱ��仯��
CH3OH(g)��ͼ1��ʾ��Ӧ�е������仯��ͼ2��ʾһ���¶��£������Ϊ1L���ܱ������м���2mol H2��һ������CO��CO��CH3OH(g)��Ũ����ʱ��仯��
 CH3OH(g)�Ļ�ѧƽ�ⳣ��Ϊ ���ﵽƽ��������������������䣬���������ѹ��Ϊ0.5L����ƽ�� �ƶ� (�������������)��
CH3OH(g)�Ļ�ѧƽ�ⳣ��Ϊ ���ﵽƽ��������������������䣬���������ѹ��Ϊ0.5L����ƽ�� �ƶ� (�������������)�� 2Fe(s)+3CO2(g) ��H =" a" kJ mol��1
2Fe(s)+3CO2(g) ��H =" a" kJ mol��1

 FeO(s) +CO(g) ��H ="a" kJ/mol
FeO(s) +CO(g) ��H ="a" kJ/mol 
 2CO2(g)+ N2(g)����H��0
2CO2(g)+ N2(g)����H��0
 N2O4(g) ��H����56.9 kJ/mol
N2O4(g) ��H����56.9 kJ/mol 
 SO3(g)+NO(g)�������Ϊ1��2��NO2��SO2���������ܱ������з���������Ӧ��������˵����Ӧ�ﵽƽ��״̬���� ��
SO3(g)+NO(g)�������Ϊ1��2��NO2��SO2���������ܱ������з���������Ӧ��������˵����Ӧ�ﵽƽ��״̬���� ��

