��Ŀ����
���û�ѧ��Ӧԭ���о��������ȡ���ȵ��ʼ��仯����ķ�Ӧ����Ҫ����
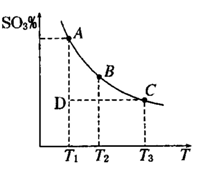
��1�����������У�SO2����������SO3�� 2SO2��g��+O2��g�� 2SO3��g���������ϵ��SO3 �İٷֺ������¶ȵĹ�ϵ����ͼ��ʾ���������κ�һ�㶼��ʾƽ��״̬��������ͼʾ�ش��������⣬
2SO3��g���������ϵ��SO3 �İٷֺ������¶ȵĹ�ϵ����ͼ��ʾ���������κ�һ�㶼��ʾƽ��״̬��������ͼʾ�ش��������⣬
��2SO2��g��+O2��g�� 2SO3��g���ġ�H 0
2SO3��g���ġ�H 0
���>����<���������ں��¡���ѹ������������ƽ����ϵ��ͨ�뺤����ƽ�� �ƶ�����������ҡ�������
�����¶�ΪT1��T2����Ӧ��ƽ�ⳣ���ֱ�ΪK1��K2����K1 K2����Ӧ���е�״̬Dʱ��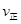
 ���>����<����=����
���>����<����=����
��2�����ǵ����Ϻ����ḻ��һ��Ԫ�أ������仯�����ڹ�ũ ҵ������������������Ҫ���ã�
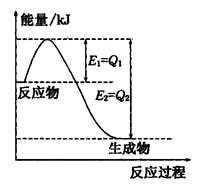
����ͼ��һ�����¶Ⱥ�ѹǿ��N2��H2��Ӧ����lmolNH3�����������仯ʾ��ͼ����д����ҵ�ϳɰ����Ȼ�ѧ����ʽ��
����H����ֵ�ú���ĸQ1��Q2�Ĵ���ʽ��ʾ��
�ڰ�������ˮ�õ���ˮ����25���£���a mol��L-1�İ�ˮ��b mol��L-1������������ϣ���Ӧ����Һ�����ԣ���c��NH4+�� c��Cl-�����>������<����=�������ú�a��b�Ĵ���ʽ��ʾ���û����Һ�а�ˮ�ĵ���ƽ�ⳣ�� .
��3����ˮ�к��д�����Ԫ�أ�����Ԫ�����ȣ���Ԫ����⣬���ں�ˮ�о��Ի���̬���ڣ���25���£���0��1L0.002mol��L-l��NaCl��Һ����μ���������0��1L0.002mol��L-l��������Һ���а�ɫ�������ɣ��ӳ����ܽ�ƽ��ĽǶȽ��Ͳ���������ԭ���� ����Ӧ�����Һ�м�������0��1L0.002mol��L-1��NaI��Һ�������������� �������������ԭ���ǣ������ӷ���ʽ��ʾ�� ��
����֪��25��ʱKSP��AgCl��=1.6��l0-10 KSP��AgI��=1.5��l0-16��

��1������������SO2����������SO3�� 2SO2��g��+O2��g��
 2SO3��g���������ϵ��SO3 �İٷֺ������¶ȵĹ�ϵ����ͼ��ʾ���������κ�һ�㶼��ʾƽ��״̬��������ͼʾ�ش��������⣬
2SO3��g���������ϵ��SO3 �İٷֺ������¶ȵĹ�ϵ����ͼ��ʾ���������κ�һ�㶼��ʾƽ��״̬��������ͼʾ�ش��������⣬��2SO2��g��+O2��g��
 2SO3��g���ġ�H 0
2SO3��g���ġ�H 0���>����<���������ں��¡���ѹ������������ƽ����ϵ��ͨ�뺤����ƽ�� �ƶ�����������ҡ�������
�����¶�ΪT1��T2����Ӧ��ƽ�ⳣ���ֱ�ΪK1��K2����K1 K2����Ӧ���е�״̬Dʱ��
 ���>����<����=����
���>����<����=������2�����ǵ����Ϻ����ḻ��һ��Ԫ�أ������仯�����ڹ�ũ ҵ������������������Ҫ���ã�

����ͼ��һ�����¶Ⱥ�ѹǿ��N2��H2��Ӧ����lmolNH3�����������仯ʾ��ͼ����д����ҵ�ϳɰ����Ȼ�ѧ����ʽ��
����H����ֵ�ú���ĸQ1��Q2�Ĵ���ʽ��ʾ��
�ڰ�������ˮ�õ���ˮ����25���£���a mol��L-1�İ�ˮ��b mol��L-1������������ϣ���Ӧ����Һ�����ԣ���c��NH4+�� c��Cl-�����>������<����=�������ú�a��b�Ĵ���ʽ��ʾ���û����Һ�а�ˮ�ĵ���ƽ�ⳣ�� .
��3����ˮ�к��д�����Ԫ�أ�����Ԫ�����ȣ���Ԫ����⣬���ں�ˮ�о��Ի���̬���ڣ���25���£���0��1L0.002mol��L-l��NaCl��Һ����μ���������0��1L0.002mol��L-l��������Һ���а�ɫ�������ɣ��ӳ����ܽ�ƽ��ĽǶȽ��Ͳ���������ԭ���� ����Ӧ�����Һ�м�������0��1L0.002mol��L-1��NaI��Һ�������������� �������������ԭ���ǣ������ӷ���ʽ��ʾ�� ��
����֪��25��ʱKSP��AgCl��=1.6��l0-10 KSP��AgI��=1.5��l0-16��
��1���٣� ���� (2��)
�ڣ� �� ��2�֣�
��2����N2(g)+3H2(g) 2NH3(g)��H=2(Q1-Q2)KJ/mol (3��)
2NH3(g)��H=2(Q1-Q2)KJ/mol (3��)
��= ��1�֣�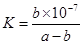 ��1�֣�
��1�֣�
��3��C(Ag+)��C(Cl-)��KSP(AgCl) ��1�֣� ��ɫ������ɻ�ɫ��1�֣�
AgCl(s)+I-(aq)=AgI(s)+Cl-(ag) ��2�֣�
�ڣ� �� ��2�֣�
��2����N2(g)+3H2(g)
 2NH3(g)��H=2(Q1-Q2)KJ/mol (3��)
2NH3(g)��H=2(Q1-Q2)KJ/mol (3��)��= ��1�֣�
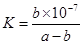 ��1�֣�
��1�֣���3��C(Ag+)��C(Cl-)��KSP(AgCl) ��1�֣� ��ɫ������ɻ�ɫ��1�֣�
AgCl(s)+I-(aq)=AgI(s)+Cl-(ag) ��2�֣�
�����������1��������ͼ��֪���¶�Խ�ߣ������ϵ��SO3�İٷֺ���ԽС��˵�������¶�ƽ�����淴Ӧ���У��������ƶ��������¶������ȷ�Ӧ�����ƶ������÷�Ӧ����ӦΪ���ȷ�Ӧ�����¡���ѹ������������ƽ����ϵ��ͨ�뺤�������Ӧ����Ӧ��������ֵ�Ũ�Ƚ��ͣ���ЧΪ����ѹǿ��ѹǿ����ƽ��������������ƶ����������ƶ���
���¶����ߣ�ƽ�������ȷ����ƶ��������淴Ӧ�ƶ���Kֵ��С��K1��K2��D״̬δ��ƽ�⣬�����ϵ��SO3�İٷֺ���С��ƽ��ʱ�ģ���Ӧ������Ӧ���У�����V����V����
��2������ͼ��֪��N2��H2��Ӧ����1molNH3�ų�������Ϊ��Q1-Q2��kJ���÷�Ӧ���Ȼ�ѧ��Ӧ����ʽΪN2(g)+3H2(g)
 2NH3(g) ��H=2(Q1-Q2)KJ/mol ��
2NH3(g) ��H=2(Q1-Q2)KJ/mol ����amol��L��1��ˮ��Һ��bmol��L��1������Һ�������Ϻ�Ӧ�����Ȼ����Һ����Һ�д��ڵ���غ�,c��NH4������c��H����=c��Cl������c��OH����,��Һ������c��H����=c��OH����,����c��NH4����=c��Cl�D��,��ͺ���Һ��c(NH3��H2O )=
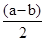 ,c��NH4����=c��Cl�D��=
,c��NH4����=c��Cl�D��=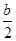 mol/L,c��H����=c��OH����=10-7mol/L,
mol/L,c��H����=c��OH����=10-7mol/L,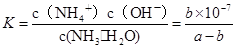 ,�𰸣�=��
,�𰸣�=��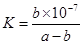
��3������Һ�����ӵ�Ũ����Qc=C(Ag+)��C(Cl-)��KSP(AgCl) ʱ���г�������������ѧʽ����ʾ���������Ӹ�������ͬ���ܶȻ�Խ���ܽ���Խ��AgCl��AgI���ܽ�ȴ��������ܽ�ȴ��ת��Ϊ�ܽ�ȸ�С�ģ�������AgClת��Ϊ�����ܵ�AgI������Ϊ��ɫ����ת��Ϊ��ɫ���������ӷ���ʽΪAgCl��s��+I���TAgI��s��+Cl����

��ϰ��ϵ�д�
�����Ŀ
 2CO2(g)+ N2(g)����H��0
2CO2(g)+ N2(g)����H��0
 N2O4(g) ��H����56.9 kJ/mol
N2O4(g) ��H����56.9 kJ/mol 
 SO3(g)+NO(g)�������Ϊ1��2��NO2��SO2���������ܱ������з���������Ӧ��������˵����Ӧ�ﵽƽ��״̬���� ��
SO3(g)+NO(g)�������Ϊ1��2��NO2��SO2���������ܱ������з���������Ӧ��������˵����Ӧ�ﵽƽ��״̬���� ��



 ���ķ�Ӧ�Ȧ�HΪ �� ��
���ķ�Ӧ�Ȧ�HΪ �� �� 2CO2(g)+ N2(g)����H��0
2CO2(g)+ N2(g)����H��0
 N2O4(g) ��H����56.9 kJ/mol
N2O4(g) ��H����56.9 kJ/mol 
 CH3OH��g������H=-90.8kJ��mol��1
CH3OH��g������H=-90.8kJ��mol��1 CH3OCH3��g��+H2O(g)ij�¶��µ�ƽ�ⳣ��Ϊ400��
CH3OCH3��g��+H2O(g)ij�¶��µ�ƽ�ⳣ��Ϊ400�� _______
_______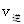 ���>������<����=������
���>������<����=������ O2(g)=HNO2(aq)+H+(aq)+H2O(1) ��H="-b" KJ/mol
O2(g)=HNO2(aq)+H+(aq)+H2O(1) ��H="-b" KJ/mol
 BaCO3(s)+SO42��(aq)
BaCO3(s)+SO42��(aq)