МвДҝДЪИЭ
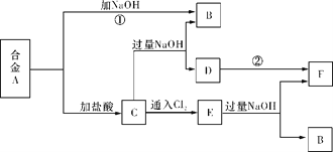
ЎҫМвДҝЎҝЕрј°Жд»ҜәПОпФЪ№ӨТөЙПУРРн¶аУГНҫЎЈТФМъЕрҝуЈЁЦчТӘіЙ·ЦОӘMg2B2O5ЎӨH2OәНFe3O4Ј¬»№УРЙЩБҝFe2O3ЎўFeOЎўCaOЎўAl2O3әНSiO2өИ)ОӘФӯБПЦЖұёЕрЛб(H3BO3)өД№ӨТХБчіМИзНјЛщКҫЈә
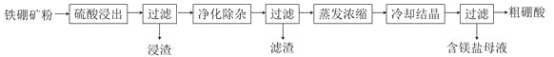
»ШҙрПВБРОКМвЈә
ЈЁ1Ј©РҙіцMg2B2O5ЎӨH2OУлБтЛб·ҙУҰөД»ҜС§·ҪіМКҪ_____________ЎЈОӘМбёЯҪюіцЛЩВКЈ¬іэККөұФцјУБтЛбЕЁ¶ИЕЁ¶ИНвЈ¬»№ҝЙІЙИЎөДҙлК©УР_________ЈЁРҙіцБҪМхЈ©ЎЈ
ЈЁ2Ј©АыУГ______өДҙЕРФЈ¬ҝЙҪ«ЖдҙУЎ°ҪюФьЎұЦР·ЦАлЎЈЎ°ҪюФьЎұЦР»№КЈУаөДОпЦККЗ______ЈЁРҙ»ҜС§КҪЈ©ЎЈ
ЈЁ3Ј©Ў°ҫ»»ҜіэФУЎұРиПИјУH2O2ИЬТәЈ¬ЧчУГКЗ_______ЎЈИ»әуФЩөчҪЪИЬТәөДpHФјОӘ5Ј¬ДҝөДКЗ________ЎЈ
ЈЁ4Ј©Ў°ҙЦЕрЛбЎұЦРөДЦчТӘФУЦККЗ___________________________ЈЁМоГыіЖЈ©ЎЈ
ЈЁ5Ј©ТФЕрЛбОӘФӯБПҝЙЦЖөГЕрЗв»ҜДЖЈЁNaBH4Ј©Ј¬ЛьКЗУР»ъәПіЙЦРөДЦШТӘ»№ФӯјБЈ¬ЖдөзЧУКҪОӘ_______ЎЈ
ЈЁ6Ј©өҘЦКЕрҝЙУГУЪЙъіЙҫЯУРУЕБјҝ№іе»чРФДЬЕрёЦЎЈТФЕрЛбәНҪрКфГҫОӘФӯБПҝЙЦЖұёөҘЦКЕрЈ¬УГ»ҜС§·ҪіМКҪұнКҫЦЖұё№эіМ___________ЎЈ
Ўҫҙр°ёЎҝMg2B2O5ЎӨH2O+2H2SO4![]() 2MgSO4+2H3BO3 јхРЎМъЕрҝу·ЫБЈҫ¶ЎўМбёЯ·ҙУҰОВ¶И Fe3O4 SiO2әНCaSO4 Ҫ«Fe2+Сх»ҜОӘFe3+ К№Al3+УлFe3+РОіЙЗвСх»ҜОп¶шіэИҘ ЈЁЖЯЛ®Ј©БтЛбГҫ
2MgSO4+2H3BO3 јхРЎМъЕрҝу·ЫБЈҫ¶ЎўМбёЯ·ҙУҰОВ¶И Fe3O4 SiO2әНCaSO4 Ҫ«Fe2+Сх»ҜОӘFe3+ К№Al3+УлFe3+РОіЙЗвСх»ҜОп¶шіэИҘ ЈЁЖЯЛ®Ј©БтЛбГҫ  2H3BO3
2H3BO3![]() B2O3+3HO B2O3+3Mg
B2O3+3HO B2O3+3Mg![]() 3MgO+2B
3MgO+2B
ЎҫҪвОцЎҝ
ТФМъЕрҝу(ЦчТӘіЙ·ЦОӘMg2B2O5H2OәНFe3O4Ј¬»№УРЙЩБҝFe2O3ЎўFeOЎўCaOЎўAl2O3әНSiO2өИ)ОӘФӯБПЦЖұёЕрЛб(H3BO3)Ј¬УЙБчіМҝЙЦӘЈ¬јУБтЛбИЬҪвЦ»УРSiO2І»ИЬЈ¬CaOЧӘ»ҜОӘОўИЬУЪЛ®өДCaSO4Ј¬Ў°ҫ»»ҜіэФУЎұРиПИјУH2O2ИЬТәЈ¬Ҫ«СЗМъАлЧУЧӘ»ҜОӘМъАлЧУЈ¬өчҪЪИЬТәөДpHФјОӘ5Ј¬К№МъАлЧУЎўВБАлЧУҫщЧӘ»ҜОӘіБөнЈ¬ФтВЛФьОӘЗвСх»ҜВБЎўЗвСх»ҜМъЈ¬И»әуХф·ўЕЁЛхЎўАдИҙҪбҫ§Ўў№эВЛ·ЦАліцH3BO3ЎЈ
(1)Mg2B2O5H2OУлБтЛб·ҙУҰөД»ҜС§·ҪіМКҪMg2B2O5H2O+2H2SO4![]() 2H3BO3+2MgSO4Ј¬ОӘМбёЯҪюіцЛЩВКЈ¬іэККөұФцјУБтЛбЕЁ¶ИЕЁ¶ИНвЈ¬»№ҝЙІЙИЎөДҙлК©УРМбёЯ·ҙУҰОВ¶И»т°СҝуОп·ЫЛй»тҪюіцКұҪБ°иЈ¬№Кҙр°ёОӘЈәMg2B2O5H2O+2H2SO4
2H3BO3+2MgSO4Ј¬ОӘМбёЯҪюіцЛЩВКЈ¬іэККөұФцјУБтЛбЕЁ¶ИЕЁ¶ИНвЈ¬»№ҝЙІЙИЎөДҙлК©УРМбёЯ·ҙУҰОВ¶И»т°СҝуОп·ЫЛй»тҪюіцКұҪБ°иЈ¬№Кҙр°ёОӘЈәMg2B2O5H2O+2H2SO4![]() 2H3BO3+2MgSO4Ј»ЙэёЯОВ¶И»т°СҝуОп·ЫЛй»тҪюіцКұҪБ°иЈ»
2H3BO3+2MgSO4Ј»ЙэёЯОВ¶И»т°СҝуОп·ЫЛй»тҪюіцКұҪБ°иЈ»
(2)АыУГFe3O4өДҙЕРФЈ¬ҝЙҪ«ЖдҙУЎ°ҪюФьЎұЦР·ЦАлЈ®Ў°ҪюФьЎұЦР»№КЈУаөДОпЦККЗSiO2ЎўCaSO4Ј¬№Кҙр°ёОӘЈәFe3O4Ј»SiO2ЎўCaSO4Ј»
(3)Ў°ҫ»»ҜіэФУЎұРиПИјУH2O2ИЬТәЈ¬ЧчУГКЗҪ«СЗМъАлЧУСх»ҜОӘМъАлЧУЈ®И»әуФЪөчҪЪИЬТәөДpHФјОӘ5Ј¬ДҝөДКЗК№МъАлЧУЎўВБАлЧУРОіЙЗвСх»ҜОпіБөн¶шіэИҘЈ¬№Кҙр°ёОӘЈәҪ«СЗМъАлЧУСх»ҜОӘМъАлЧУЈ»К№МъАлЧУЎўВБАлЧУРОіЙЗвСх»ҜОпіБөн¶шіэИҘЈ»
(4)ЧоәуЕЁЛхҪбҫ§КұБтЛбГҫТЧҪбәПЛ®ТФҫ§МеОціцЈ¬ФтЎ°ҙЦЕрЛбЎұЦРөДЦчТӘФУЦККЗБтЛбГҫЈ¬№Кҙр°ёОӘЈәБтЛбГҫЈ»
(5)NaBH4ОӘАлЧУ»ҜәПОпЈ¬ә¬АлЧУјьЎў№ІјЫјьЈ¬ЖдөзЧУКҪОӘ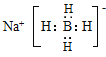 Ј¬№Кҙр°ёОӘЈә
Ј¬№Кҙр°ёОӘЈә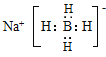 Ј»
Ј»
(6)ТФЕрЛбәНҪрКфГҫОӘФӯБПҝЙЦЖұёөҘЦКЕрөД»ҜС§·ҪіМКҪОӘ2H3BO3![]() B2O3+3H2OЎўB2O3+3Mg
B2O3+3H2OЎўB2O3+3Mg![]() 2B+3MgOЈ¬ЧЬ·ҙУҰОӘ2H3BO3+3Mg
2B+3MgOЈ¬ЧЬ·ҙУҰОӘ2H3BO3+3Mg ![]() 3MgO+2B+3H2OЈ¬№Кҙр°ёОӘЈә2H3BO3+3Mg
3MgO+2B+3H2OЈ¬№Кҙр°ёОӘЈә2H3BO3+3Mg![]() 3MgO+2B+3H2OЎЈ
3MgO+2B+3H2OЎЈ

 МфХҪ100өҘФӘјмІвКФҫнПөБРҙр°ё
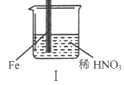
МфХҪ100өҘФӘјмІвКФҫнПөБРҙр°ёЎҫМвДҝЎҝФЪНЁ·зічЦРҪшРРПВБРКөСйЈә
ІҪЦи |
|
| |
ПЦПу | FeұнГжІъЙъҙуБҝОЮЙ«ЖшЕЭЈ¬ТәГжЙП·ҪұдОӘәмЧШЙ« | FeұнГжІъЙъЙЩБҝәмЧШЙ«ЖшЕЭәуЈ¬СёЛЩНЈЦ№ | FeЎўCuҪУҙҘәуЈ¬ЖдұнГжҫщІъЙъәмЧШЙ«ЖшЕЭ |
ПВБРЛө·ЁІ»ХэИ·өДКЗ
A. IЦРЖшМеУЙОЮЙ«ұдәмЧШЙ«өД»ҜС§·ҪіМКҪЈә2NO+O2ЈҪ2NO2
B. IIЦРөДПЦПуЛөГчFeұнГжРОіЙЦВГЬөДСх»ҜІгЈ¬ЧиЦ№FeҪшТ»ІҪ·ҙУҰ
C. ¶ФұИIЎўIIЦРПЦПуЈ¬ЛөГчПЎHNO3өДСх»ҜРФЗҝУЪЕЁHNO3
D. Хл¶ФўуЦРПЦПуЈ¬ФЪFeЎўCuЦ®јдБ¬ҪУөзБчјЖЈ¬ҝЙЕР¶ПFeКЗ·сұ»Сх»Ҝ
ЎҫМвДҝЎҝПВБРёчЧйОпЦКЦРЈ¬ВъЧгПВНјОпЦКТ»ІҪЧӘ»Ҝ№ШПөөДСЎПоКЗ
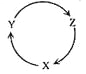
СЎПо | X | Y | Z |
A | Na | NaOH | NaHCO3 |
B | Cu | CuSO4 | Cu(OH)2 |
C | C | CO | CO2 |
D | Si | SiO2 | H2SiO3 |
A.AB.BC.CD.D
ЎҫМвДҝЎҝПВұнёчЧйОпЦКЦРЈ¬ОпЦКЦ®јдІ»ҝЙДЬКөПЦИзНјЛщКҫЧӘ»ҜөДКЗЈЁ Ј©
![]()
СЎПо | X | Y | Z | M |
A | NH3 | NO | NO2 | O2 |
B | Cl2 | FeCl3 | FeCl2 | Fe |
C | Al | AlЈЁOHЈ©3 | NaAlO2 | NaOH |
D | NaOH | Na2CO3 | NaHCO3 | CO2 |
A.AB.BC.CD.D