��Ŀ����
����Ŀ��(1)��ӦFe(s)��CO2(g)![]() FeO(s)��CO(g)����H1��ƽ�ⳣ��ΪK1����ӦFe(s)��H2O(g)
FeO(s)��CO(g)����H1��ƽ�ⳣ��ΪK1����ӦFe(s)��H2O(g)![]() FeO(s)��H2(g)����H2��ƽ�ⳣ��ΪK2���ڲ�ͬ�¶�ʱK1��K2��ֵ���±���
FeO(s)��H2(g)����H2��ƽ�ⳣ��ΪK2���ڲ�ͬ�¶�ʱK1��K2��ֵ���±���
700 �� | 900 �� | |
K1 | 1.47 | 2.15 |
K2 | 2.38 | 1.67 |
�ٷ�ӦCO2(g)��H2(g)![]() CO(g)��H2O(g)����H��ƽ�ⳣ��ΪK������H��________(����H1����H2��ʾ)��K��________(��K1��K2��ʾ)���������������֪����ӦCO2(g)��H2(g)
CO(g)��H2O(g)����H��ƽ�ⳣ��ΪK������H��________(����H1����H2��ʾ)��K��________(��K1��K2��ʾ)���������������֪����ӦCO2(g)��H2(g)![]() CO(g)��H2O(g)��_____________________��Ӧ(��������������������)��
CO(g)��H2O(g)��_____________________��Ӧ(��������������������)��
�����ж�CO2(g)��H2(g)![]() CO(g)��H2O(g)�ﵽ��ѧƽ��״̬��������_______(����ĸ)��
CO(g)��H2O(g)�ﵽ��ѧƽ��״̬��������_______(����ĸ)��
A��������ѹǿ���䡡�� B�����������c(CO)����
C��v��(H2)��v��(H2O) D��c(CO)��c(CO2)
(2)һ���¶��£���ij�ܱ������м����������۲�����һ������CO2���壬������Ӧ��Fe(s)��CO2(g)![]() FeO(s)��CO(g)����H>0��CO2��Ũ����ʱ��Ĺ�ϵ��ͼ��ʾ��
FeO(s)��CO(g)����H>0��CO2��Ũ����ʱ��Ĺ�ϵ��ͼ��ʾ��

�ٸ������·�Ӧ��ƽ�ⳣ��Ϊ______��������������CO2����ʼŨ��Ϊ2.0 mol��L��1����ƽ��ʱCO2��Ũ��Ϊ______mol��L��1��
�����д�ʩ����ʹƽ��ʱ �������________(����ĸ)��
�������________(����ĸ)��
A�������¶� B������ѹǿ
C������һ������CO2 D���ټ���һ��������
���𰸡���H1����H2  ���� BC 2.0�� 0.67(��2/3) A
���� BC 2.0�� 0.67(��2/3) A
��������
��1���ٸ��ݸ�˹���ɣ������ڵ���CO2(g)��H2(g)![]() CO(g)��H2O(g)����H����H1����H2����ӦFe(s)��CO2(g)
CO(g)��H2O(g)����H����H1����H2����ӦFe(s)��CO2(g)![]() FeO(s)��CO(g)��ƽ�ⳣ��K1��c(CO)/c(CO2)����ӦFe(s)��H2O(g)
FeO(s)��CO(g)��ƽ�ⳣ��K1��c(CO)/c(CO2)����ӦFe(s)��H2O(g)![]() FeO(s)��H2(g)��ƽ�ⳣ��K2��c(H2)/c(H2O)����ӦCO2(g)��H2(g)
FeO(s)��H2(g)��ƽ�ⳣ��K2��c(H2)/c(H2O)����ӦCO2(g)��H2(g)![]() CO(g)��H2O(g)��ƽ�ⳣ��K��c(CO)��c(H2O)/c(CO2)��c(H2)��
CO(g)��H2O(g)��ƽ�ⳣ��K��c(CO)��c(H2O)/c(CO2)��c(H2)��![]() �����ݱ������ݿ�֪���¶ȵ����ߣ�K1����K2��С������Kֵ���¶����߶�������˷�ӦCO2(g)��H2(g)
�����ݱ������ݿ�֪���¶ȵ����ߣ�K1����K2��С������Kֵ���¶����߶�������˷�ӦCO2(g)��H2(g)![]() CO(g)��H2O(g)�����ȷ�Ӧ����A��÷�Ӧ������ѹǿΪ������ѹǿ���䲻һ��ƽ�⣬��A������B����������c(CO)���䣬һ���ﵽƽ��״̬����B��ȷ��C�v��(H2)��v��(H2O)= v��(H2)����Ϊƽ��״̬����C��ȷ��D�c(CO)��c(CO2)����˵��v����v��������˵����Ũ�Ȳ��ٷ����仯����һ��ƽ�⣬��D������
CO(g)��H2O(g)�����ȷ�Ӧ����A��÷�Ӧ������ѹǿΪ������ѹǿ���䲻һ��ƽ�⣬��A������B����������c(CO)���䣬һ���ﵽƽ��״̬����B��ȷ��C�v��(H2)��v��(H2O)= v��(H2)����Ϊƽ��״̬����C��ȷ��D�c(CO)��c(CO2)����˵��v����v��������˵����Ũ�Ȳ��ٷ����仯����һ��ƽ�⣬��D������
(2)��ͼ�����߿ɵã�ƽ��ʱ������̼��Ũ��Ϊ0.5 mol��L��1�����Ķ�����̼��Ũ��Ϊ1 mol��L��1�����ݻ�ѧ����ʽ���ﵽƽ��ʱ��c(CO)��1 mol��L��1���������·�Ӧ��ƽ�ⳣ��K��c(CO)/c(CO2)��(1 mol��L��1)/(0.5 mol��L��1)��2.0��������������CO2����ʼŨ��Ϊ2.0 mol��L��1��������ʽ���£�
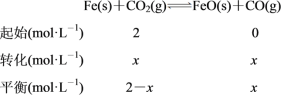
��Ϊ�¶�û��������K��2����x��(2��x)��2�����x��4/3������ƽ��ʱCO2��Ũ��Ϊ(2��4/3) mol��L��1��2/3 mol��L��1��
��A�����Ӧ���ȣ������¶ȣ�ƽ�������ƶ�����![]() ����A��ȷ��B���Ӧǰ��������䣬����ѹǿƽ�ⲻ�ƶ���
����A��ȷ��B���Ӧǰ��������䣬����ѹǿƽ�ⲻ�ƶ���![]() ���䣻��B����C��ٳ���һ������CO2������ƽ�ⳣ�����䣬��
���䣻��B����C��ٳ���һ������CO2������ƽ�ⳣ�����䣬��![]() ���䣬��C����D��ټ���һ�������ۣ�ƽ�ⲻ�ƶ���
���䣬��C����D��ټ���һ�������ۣ�ƽ�ⲻ�ƶ���![]() ���䣬��D�����������������������ѡ��ΪA��
���䣬��D�����������������������ѡ��ΪA��

����Ŀ��������������(FeC2O4��xH2O)Ϊ����ɫ��ĩ��������ˮ������������Ӱ������ҩ��ҵ��ij��ѧ��ȤС��������ʽ�������̽�����ش��������⣺
����̽��
ѡ�������Լ����ʵ�鷽��������±����ݡ�
�Լ�������KMnO4 ��Һ��H2O2 ��Һ��KSCN ��Һ
���� | ���� | ��������� |
(1)ȡ�������������������Թ��У�����2mL ˮ���� �� | �е���ɫ�������ϲ���Һ��ɫ | ___________________________ |
(2)��������2mLϡ���ᣬ�� | �����ܽ⣬��Һ��Ϊdz��ɫ | ���������������ᣬ��������____����(����ǿ������������������������) |
(3)����(2)������Һ�еμӼ���K3[Fe(CN)6]��Һ | ___________ | ����Fe2+ |
(4)________________________________ | ___________ | H2C2O4 ��C2O42-���л�ԭ�� |
����̽�����ζ�ʵ���x ��ֵ
(5)�ζ�ǰ�����в�������ȷ˳����c�� _________ ��d(����ĸ���)��
a���ž��ζ��ܼ�������ݲ�����Һ�� b��ʢװ0.1000 mol��L-1 ������KMnO4 ��Һ
c����©����ϴ d.��ʼ��������¼Ϊ0.50 mL
e.��0.1000 mol��L-1 ������KMnO4 ��Һ��ϴ
(6)��ȡ m ����Ʒ����������ϡ�����ܽ⣬�ò���(5)���ı�KMnO4 ��Һֱ�ӵζ����ܽ�ʱ�����ϵIJ���������_______________ ���ζ���Ӧ������������________________��
(7)�յ����Ϊ20.50mL���������ʵ���������x��____________(�ú�m �Ĵ���ʽ��ʾ��FeC2O4 ����Է�������Ϊ144)��