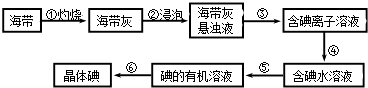
��Ŀ����
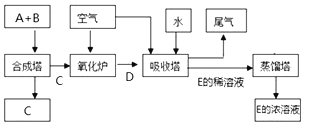
����Ŀ����ͼΪij��ҵ�����Ļ������̣�����A��BΪ���ʣ�����Ϊ�������A��������������塣����D�ڽ����������������ɫ��Ϊ����ɫ����ش��������⣺
��1����д�����ǵĻ�ѧʽ��
C:___________E:___________
��2��Ϊ�˾����ܼ���β�����ŷţ�������������ڵ�����D���������������Ϊ_____________
��3��E��ϡ��Һ��ֱ������ķ��������õ�70%���ϵ�Ũ��Һ�ģ����Ϊ�����E��Һ��Ũ�ȣ�ͨ�����������м�����������ˮ���������ڴ˵���ˮ��Ϊ��____________��ֻ��һ�ּ��ɣ�������ˮ�����ŵ��ȱ������Щ____________________��
��4��������ݲ��лẬ�д�����ij�°��ʵ������Ϊ�о������ʣ�������D��ij����ɫ�����ﷴӦ��ȡ���°������ȡʱ�����Ļ�ѧ��Ӧ����ʽΪ_________________________�����÷�Ӧ���б����4.48LD���뷴Ӧ����Ӧ������ת�Ƶ�����Ŀ��________________
���𰸡� NH3 HNO3 4:15 Ũ���� �������豸��ʴ�Ƚ����� 2NO+Na2O2=2NaNO2 0.2NA����1.204��1023��
��������A��BΪ���ʣ�����Ϊ�������A�����������������A������������D�ڽ����������������ɫ��Ϊ����ɫ�����D��NO��B�ǵ�����C�ǰ�����E�����ᡣ
��1���������Ϸ�����֪C��NH3��E��HNO3����2������4NO+3O2+2H2O=4HNO3��֪NO������������������4��3�������ڿ�����Լռ20%�����Խ����������ڵ�����NO���������������Ϊ4:15����3��Ũ���������ˮ�ԣ���˳����ڴ˵���ˮ��ΪŨ���ᣬ����Ũ������и�ʴ�ԣ�����ȱ���Ƕ������豸��ʴ�Ƚ���������4������ɫ�������ǹ������ƣ���NO��Ӧ�����������ƣ�����ʽΪ2NO+Na2O2=2NaNO2����Ӧ�е�Ԫ�ػ��ϼ۴�+2�����ߵ�+3�ۣ�ʧȥ1��������4.48L��״����NO��0.2mol�����Է�Ӧ������ת�Ƶ�����Ŀ��0.2NA��