��Ŀ����
20��ij�ᾧˮ����A�������������Ӻ�һ�������ӣ���ȡ����������Ϊ1.96g��A�ֱ��Ƴ���Һ��һ�ݼ�������Ba��OH��2��Һ�����ɰ�ɫ�������漴������Ϊ����ɫ�������к��ɫ�����ȸû����ݳ�0.224L����״������ʹʪ��ĺ�ɫʯ����ֽ����������B����ϡ���ᴦ���������ϴ�Ӻ���õ�2.33g��ɫ����C����һ�ݼ��뺬0.001mol��KMnO4��������Һ��MnO4-��ǡ����ȫ����ԭΪMn2+����ش��������⣺��1���ᾧˮ����A�Ļ�ѧʽ����NH4��2Fe��SO4��2•6H2O[��NH4��2SO4•FeSO4•6H2O]��B�ĵ���ʽΪ
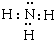 ��
����2���û�ѧ����ʽ��ʾ��ɫ����������ɫ�����ɫ��Ӧ��Ӧ4Fe��OH��2+O2+2H2O=4Fe��OH��3��
��3��������A����������һ��������ԭ�����ڶ��������г������궨�ظ���ػ���������Һ���Է�����A��������������ԭ��A
A�������б����������ȶ� B����ԭ�Ը�ǿ
��4��A����ˮ��Һ����������ˮ��������ԣ��ڻ�ѧ����ˮ������ʺܶ࣬�����Ȼ��ᣨClSO3H�� ����һ������ˮ���һԪǿ�ᣬ��д��ClSO3H������NaOH��Һ��Ӧ�Ļ�ѧ����ʽClSO3H+3NaOH=NaCl+Na2SO4+2H2O��
���� һ�ݼ�������Ba��OH��2��Һ�����ɰ�ɫ�������漴������Ϊ����ɫ�������к��ɫ��˵������Fe2+���ӣ����ȸû����ݳ�0.224L����״������ʹʪ��ĺ�ɫʯ����ֽ����������BΪNH3��˵������NH4+����ϡ���ᴦ���������ϴ�Ӻ���õ���ɫ����CΪBaSO42.33g��˵������SO42-����������Ϣ��֪1.96g�ĸýᾧˮ�����У�n��NH4+��=$\frac{0.224L}{22.4L/mol}$=0.01mol������Ϊ��m��NH4+��=0.01mol��18g/mol=0.18g��n��SO42-��=$\frac{2.33g}{233g/mol}$=0.01mol������Ϊ��m��SO42-��=0.01mol��96g/mol=0.96g��KMnO4��������Һ���������ԣ���Fe2+����������ԭ��Ӧ�����ݵ�ʧ���ӵ����ʵ�����ȣ����У�0.001mol����7-2��=n����3-2�������ԣ�n=0.005mol����Fe2+�����ʵ���Ϊ0.005mol������Ϊ��0.005mol��56g/mol=0.28g�����Խᾧˮ������ˮ������Ϊ��1.96g-0.18g-0.96g-0.28g=0.54g��n��H2O��=$\frac{0.54g}{18g/mol}$=0.03mol�����ԣ�1.96g�ĸýᾧˮ�����У�m��NH4+����n��Fe2+����n��SO42-����n��H2O��=0.01mol��0.005mol��0.01mol��0.03mol=2��1��2��6������A�Ļ�ѧʽΪ����NH4��2Fe��SO4��2•6H2O��[��NH4��2SO4•FeSO4•6H2O]���ݴ˴��⣮
��� �⣺һ�ݼ�������Ba��OH��2��Һ�����ɰ�ɫ�������漴������Ϊ����ɫ�������к��ɫ��˵������Fe2+���ӣ����ȸû����ݳ�0.224L����״������ʹʪ��ĺ�ɫʯ����ֽ����������BΪNH3��˵������NH4+����ϡ���ᴦ���������ϴ�Ӻ���õ���ɫ����CΪBaSO42.33g��˵������SO42-����������Ϣ��֪1.96g�ĸýᾧˮ�����У�n��NH4+��=$\frac{0.224L}{22.4L/mol}$=0.01mol������Ϊ��m��NH4+��=0.01mol��18g/mol=0.18g��n��SO42-��=$\frac{2.33g}{233g/mol}$=0.01mol������Ϊ��m��SO42-��=0.01mol��96g/mol=0.96g��KMnO4��������Һ���������ԣ���Fe2+����������ԭ��Ӧ�����ݵ�ʧ���ӵ����ʵ�����ȣ����У�0.001mol����7-2��=n����3-2�������ԣ�n=0.005mol����Fe2+�����ʵ���Ϊ0.005mol������Ϊ��0.005mol��56g/mol=0.28g�����Խᾧˮ������ˮ������Ϊ��1.96g-0.18g-0.96g-0.28g=0.54g��n��H2O��=$\frac{0.54g}{18g/mol}$=0.03mol�����ԣ�1.96g�ĸýᾧˮ�����У�m��NH4+����n��Fe2+����n��SO42-����n��H2O��=0.01mol��0.005mol��0.01mol��0.03mol=2��1��2��6������A�Ļ�ѧʽΪ����NH4��2Fe��SO4��2•6H2O��[��NH4��2SO4•FeSO4•6H2O]��
��1����������ķ�����֪���ᾧˮ����AΪ��NH4��2Fe��SO4��2•6H2O[��NH4��2SO4•FeSO4•6H2O]��BΪNH3�������ʽΪ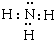 ��
��
�ʴ�Ϊ����NH4��2Fe��SO4��2•6H2O[��NH4��2SO4•FeSO4•6H2O]��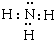 ��
��
��2���û�ѧ����ʽ��ʾ��ɫ����������ɫ�����ɫ��Ӧ��ӦΪ4Fe��OH��2+O2+2H2O=4Fe��OH��3��
�ʴ�Ϊ��4Fe��OH��2+O2+2H2O=4Fe��OH��3��
��3�����ڣ�NH4��2Fe��SO4��2•6H2O�ڿ����б����������ȶ��������ڶ��������г������궨�ظ���ػ���������Һ���ã�NH4��2Fe��SO4��2•6H2O����������������
�ʴ�Ϊ��A��
��4��ClSO3H������NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪClSO3H+3NaOH=NaCl+Na2SO4+2H2O��
�ʴ�Ϊ��ClSO3H+3NaOH=NaCl+Na2SO4+2H2O��
���� ���⿼�����ӵļ�����ƶϣ���Ŀ�Ѷ��еȣ�ע����غ�ĽǶ��ƶϽᾧˮ����Ļ�ѧʽ��

| A�� | [H+]=[OH-]����Һ | B�� | pH��7����Һ | ||
| C�� | [H+]=1.0��10-7mol•L-1 ����Һ | D�� | [H+]��[OH-]����Һ |
| A�� | ���������ʻ��ã�����������Ϻ�����������ը | |
| B�� | ����HCl�������Ƿ����Cl2�����ǽ�����ͨ����������Һ | |
| C�� | ŨHCl����MnO2������ȡCl2��ʵ����ֻ��ԭ�������� | |
| D�� | ʵ������ȡ����ʱ��Ϊ�˷�ֹ������Ⱦ���������������������������Һ���� |
| A�� | ����20 mL | B�� | ��20 mL | C�� | ����20mL | D�� | ����5mL |
| A�� | ���׳�ƻ��ɽ��ܵ� | B�� | �ٿ��������������г����������� | ||
| C�� | �÷����������������� | D�� | �Ѹߺ��ܲ�ҵת�Ƶ�Ƿ������� |
| ѡ�� | ��ѧ��Ӧ�������ӷ���ʽ | ���� |
| A | ����ϡ���ᷴӦ�� 2Fe+6H+�T2Fe3++3H2�� | ��ȷ |
| B | ����ʯ���ڴ���ķ�Ӧ�� CaCO3+2H+�TCa2++CO2��+H2O | ������ӦдΪ������ʽCH3COOH��CaCO3Ӧд��������ʽ |
| C | FeCl3��Һ����ʴ��ͭ���� Fe3++Cu�TFe2++Cu2+ | ��ȷ |
| D | NH4HCO3��Һ�����NaOHŨ��Һ���ȣ�NH4++OH-$\frac{\underline{\;\;��\;\;}}{\;}$NH3��+H2O | ����HCO3-Ҳ������OH-��Ӧ |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ��ҵұ����Fe2O3+3CO$\frac{\underline{\;����\;}}{\;}$2Fe+3CO2 | |
| B�� | ����ͭCu2S+O2$\frac{\underline{\;����\;}}{\;}$2Cu+SO2 | |
| C�� | ��ҵұ��þMgCl2$\frac{\underline{\;���\;}}{\;}$Mg+Cl2 | |
| D�� | ��ҵұ����Al2O3+2Fe$\frac{\underline{\;����\;}}{\;}$Fe2O3+2Al |
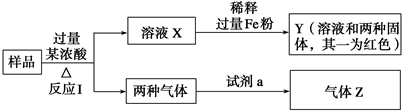
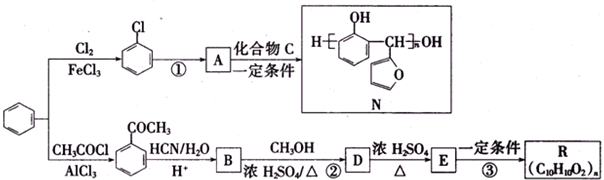
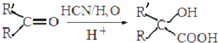
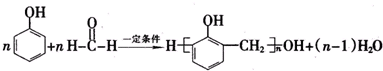
 ��
�� ��
��