��Ŀ����
1������Ũ�Ⱦ�Ϊ0.1mol/L��������Һ�������ᡡ�ڴ��ᡡ���������ơ����Ȼ����ش��������⣺
��1���٢ڢۢ�������Һ����ˮ�������H+Ũ���ɴ�С��˳���ǣ�����ţ��ܢڢۢ٣�
��2�����ۺܵ͢������Ϻ��Һ�и�����Ũ���ɴ�С��˳����c��Na+��=c��Cl-����c��OH-����c��NH4+����c��H+��
��3����֪t��C��KW=1��10-13����t��C�������������������=����25��C��
��4��25��Cʱ����pH=x�������pH=y������������Һ��x��6��y��8����ȡa L��������b L������������Һ��Ӧ��ǡ����ȫ�кͣ�����Һ��pH��x��y���Ĺ�ϵʽΪ$\frac{a}{b}$=10x+y-14�������ʽ����
���� ��1�����������������H2O�ĵ��룬����Һ��H+��OH-Ũ��Խ���ε�ˮ���ܴٽ�ˮ�ĵ��������
��2�����ݶ�����ȫ��Ӧ����NaCl��NH3•H2O��Һ������
��3��ˮ�ĵ��������ȷ�Ӧ�������¶ȴٽ�ˮ���룻
��4��������һԪǿ�ᣬ����������һԪǿ���ǡ����ȫ�кͣ���Һ�����ԣ������������������������ʵ�����ͬ���㣮
��� �⣺��1������Һ�м��������������H2O�ĵ��룬����Һ��H+��OH-Ũ��Խ����ˮ�������H+Ũ��ԽС���ε�ˮ���ܴٽ�ˮ�ĵ��룬������ˮ�����c��H+���ɴ�С��˳��Ϊ���ܢڢۢ٣�
�ʴ�Ϊ���ܢڢۢ٣�
��2��������ȫ��Ӧ����NaCl��NH3•H2O������NH3•H2O��������������ӣ�������Һ�ʼ��ԣ���Һ������Ũ�ȴ�С��ϵΪ��c��Na+��=c��Cl-����c��OH-����c��NH4+����c��H+����
�ʴ�Ϊ��c��Na+��=c��Cl-����c��OH-����c��NH4+����c��H+����
��3��ˮ�ĵ��������ȷ�Ӧ�������¶ȴٽ�ˮ���룬��ˮ�����ӻ���������t��ʱ��Kw=1��10-13��10-14������t�棾25�棬�ʴ�Ϊ������
��4��������һԪǿ�ᣬ����������һԪǿ���ǡ����ȫ�кͣ���Һ�����ԣ������������������������ʵ�����ͬ��֪��aL��10-xmol/L=bL��10-14+ymol/L��
$\frac{a}{b}$=10x+y-14���ʴ�Ϊ��$\frac{a}{b}$=10x+y-14��
���� ���⿼����ˮ�ĵ��뼰��Һ������Ũ�ȴ�С�ıȽϣ���Ŀ�Ѷ��еȣ�ע���¶�Ӱ��ˮ�����ӻ����¶Ȳ�ͬ��ˮ�����ӻ���ͬ��

 ��ڽ��ȫ������ϵ�д�
��ڽ��ȫ������ϵ�д�| A�� | ����ᴿ��Ӧ�ã��ٽ��˰뵼��Ԫ���뼯��оƬ�ķ�չ | |
| B�� | ˮ�ࡢ���������϶��Ǹ߷��Ӳ��� | |
| C�� | �ֻ���������ͨ��������Ҫ�ɷֲ�ͬ | |
| D�� | ͨ������£����Ͻ��ڿ����в��ױ���ʴ���������������� |
��1��PM2.5������������Ļ�������ж��°����ʣ����Ԫ�ط���ΪAs��λ��Ԫ�����ڱ��������ڵڢ�A�壮
��2����PM2.5����������ˮ�����ɴ���Һ���������ˮ���������ӵĻ�ѧ��ּ���ƽ��Ũ�����±���
| ���� | K+ | Na+ | NH4+ | SO42- | NO3- | Cl- |
| Ũ��/mol•L-1 | 4��10-6 | 6��10-6 | 2��10-5 | 4��10-5 | 3��10-5 | 2��10-5 |
��3�������£���Na2SO3��Һ����SO2�Ĺ����У�pH��n��SO32-����n��HSO3-���仯���±���
| N��SO32-����n��HSO3-�� | 91��9 | 1��1 | 9��91 |
| pH | 8.2 | 7.2 | 6.2 |
��4����֪��2NO��g��+O2��g��?2NO2��g����H=-113.0kJ•mol-1
2SO2��g��+O2��g��?2SO3��g����H=-196.6kJ•mol-1
��ӦNO2��g��+SO2��g��?SO3��g��+NO��g���ġ�H=-41.8kJ•mol-1��
һ�������£���NO��SO2�������1��2�����ܱ������з���������Ӧ��������˵����Ӧ�ﵽƽ��״̬����b��
a����ϵѹǿ���ֲ��� b�����������ɫ���ֲ��� c��SO3��NO��������ֲ��� d��ÿ����1molSO3��ͬʱ����1molNO2��
| Ԫ�ش��� | X | Y | Z | M | R |
| ԭ�Ӱ뾶/nm | 0.186 | 0.102 | 0.075 | 0.074 | 0.143 |
| ��Ҫ���ϼ� | +1 | +6-2 | +5-3 | -2 | +3 |
| A�� | ���Ӱ뾶��С��Y2-��M2-��R3+ | |
| B�� | Y��M��ɵĻ��������γ������ԭ��֮һ | |
| C�� | ��YM2ͨ��BaCl2��Һ���д�����ɫ�������� | |
| D�� | X������������ˮ������Դ���R����������ˮ������� |
 SO42-+CaCO3��
SO42-+CaCO3��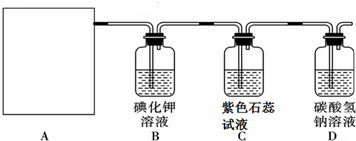
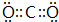 ��
�� ��
�� ������һ����Ҫ���л�����ԭ�ϣ������������������գ�
������һ����Ҫ���л�����ԭ�ϣ������������������գ� $\stackrel{����}{��}$
$\stackrel{����}{��}$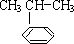 ����Ӧ����Ϊ�ӳɷ�Ӧ
����Ӧ����Ϊ�ӳɷ�Ӧ