��Ŀ����
����Ŀ������̿�������ǹ�ҵ������Ҫ����֮һ�����������£�
![]()
����������⣺
��1�����������£�NaNO2��Һֻ�ܽ� I������ΪI2��ͬʱ����NO��д����Ӧ�ٵ����ӷ���ʽ���������ת�Ƶ���Ŀ�ͷ���______________��
��2��������I2�⾭��������ת��ΪI����IO3����ת��ΪI2�Ĺ��̣���������Ŀ����_______��
��3����Ӧ�ڷ���ʱ�����ڵ���ˮ���ܽ�Ȳ�����Һ�ײ��й������ɣ����ڷ�Ӧ���ȣ���ʱ��Һ�Ϸ�����_____������ɫ�������塣��ˣ���Ӧ����Ҫ��______�����½��С�
��4��ʵ���Ҵӷ�Ӧ��������Һ��ȡ�⣬�ɼ���CCl4_______����������ƣ��⣬���ѵ��ˮ��Һ����ȡ����������________�����������ƣ�����������Һ��
��5������̿��������I2Ҳ������NaHSO3����ת��ΪI�����÷�Ӧ����������Ϊ_______���������ţ���
��֪NaHSO3��Һ�������ԣ��Դ�ƽ��ĽǶȽ���ԭ��____________��
��0.1mol/L��NaHSO3��Һ�м��백ˮ�����ԣ����жϣ�c(Na+)____c(SO32�C)+ c(HSO3�C)+ c(H2SO3)������>������<������=������
���𰸡�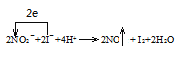 ������Ũ�ȣ������� ��ɫ ��ˮԡ���ߵ��� ��ȡ ��Һ©�� SO42�� NaHSO3��Һ��HSO3�C����ˮ���Լ��ԣ����ܵ����H+ �����ԣ����������̶ȴ���ˮ��̶ȣ�����NaHSO3��Һ�������� =
������Ũ�ȣ������� ��ɫ ��ˮԡ���ߵ��� ��ȡ ��Һ©�� SO42�� NaHSO3��Һ��HSO3�C����ˮ���Լ��ԣ����ܵ����H+ �����ԣ����������̶ȴ���ˮ��̶ȣ�����NaHSO3��Һ�������� =
��������
(1)�������ƾ��������ԣ������Ӿ��л�ԭ�ԣ����������£����߷���������ԭ��Ӧ����һ�������͵��ˮ���ݴ˷������
(2)�����е�Ԫ�ؾ�����I2��I-��IO3-��I2�Ĺ��̿���������Ũ�ȣ�
(3)����ˮ�е��ܽ�Ȳ�������������
(4)����ˮ�е��ܽ�Ȳ����������л��ܼ���
(5)I2������NaHSO3����������ԭ��Ӧ��NaHSO3�������������ƣ�NaHSO3��Һ��HSO3�C����ˮ�����ܵ��룻���������غ�������ݴ˷������
(1)�������ƾ��������ԣ������Ӿ��л�ԭ�ԣ����������£����߷���������ԭ��Ӧ����һ�������͵��ˮ�����ӷ���ʽΪ��2NO2-+4H++2I-�T2NO��+I2+2H2O������ת�Ƶ���Ŀ�ͷ�����Ա�ʾΪ![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��
(2)�����У���Ԫ�ؾ�����I2��I-��IO3-��I2�ı仯���̣�����������ԭ���ǿ���������Ũ�ȣ��ﵽ������Ԫ�ص�Ŀ�ģ��ʴ�Ϊ��������Ԫ�أ�
(3)��Ӧ�ڷ���ʱ����Һ�ײ����Ϻ�ɫ�Ĺ������ɣ���������������ʱ��Һ�Ϸ�������ɫ�ĵ����������ԣ���Ӧ����Ҫ�ڱ�ˮԡ���ߵ��������½��У��ʴ�Ϊ����ɫ (4). ��ˮԡ���ߵ��£�
(4)ʵ���Ҵӷ�Ӧ��������Һ��ȡ�⣬����ˮ�е��ܽ�Ȳ����������л��ܼ����ɼ���CCl4��ȡ�⣬���ѵ��ˮ��Һ����ȡ��������ȡ���÷�Һ©������������Һ���ʴ�Ϊ����ȡ����Һ©����
(5)����̿��������I2Ҳ������NaHSO3����ת��ΪI������ΪNaHSO3�ܹ������������������ƣ���˸÷�Ӧ����������ΪSO42����NaHSO3��Һ��HSO3�C����ˮ�����ܵ��룬ˮ��ʹ���Լ��ԣ�����ʹ�������ԣ����������̶ȴ���ˮ��̶ȣ�����NaHSO3��Һ�������ԣ���0.1mol/L��NaHSO3��Һ�м��백ˮ�����ԣ���Һ��c(H+)=c(OH-)�����������غ���c(Na+)=c(SO32-)+c(HSO3-)+c(H2SO3)���ʴ�Ϊ��SO42����NaHSO3��Һ��HSO3�C����ˮ���Լ��ԣ����ܵ����H+ �����ԣ����������̶ȴ���ˮ��̶ȣ�����NaHSO3��Һ�������ԣ�=��









