��Ŀ����
����Ŀ����ѧ��������������������أ������ѧ����ѧ֪ʶ�����������
(1)����������ұ����Ӧ����ʱ���õ�ⷨ����___________��
a��Cu2S b��NaCl c��Fe2O3 d��HgS
(2) �����鶾�����ҹ�����ǧ����ʷ��������Ҫ���ڼ����Ƿ��к���Ԫ�ص��ж����ʡ��䷴Ӧԭ��֮һΪ��4Ag��2H2S��O2=2Ag2S��2H2O���������ɫ��������ʢ��ʳ��ˮ������������һ��ʱ����ɸ�ԭ��ԭ�����γ���ԭ��أ���ԭ��صĸ�����Ӧ��Ϊ��______��
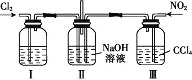
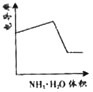
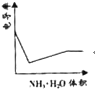
(3)ijͬѧ���һ��ȼ�ϵ��(����ͼ��ʾ)��Ŀ����̽���ȼҵԭ���ʹ�ͭ�ľ���ԭ����������װ����XΪ�����ӽ���Ĥ��
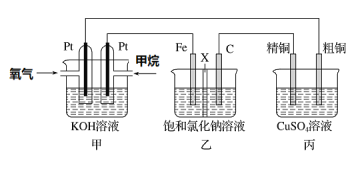
��ͨ�����ĵ缫Ϊ________(��������������������)���õ缫��ӦʽΪ___________��
����װ�ù���һ��ʱ���ϻ�ѧ����������缫���������̪����ԭ��____________��
�������ͭ�к���п���������ʣ���װ���з�Ӧһ��ʱ�䣬����ͭ��ҺŨ�Ƚ�________(��������������С������������)����ͭ�缫�ϵĵ缫��ӦʽΪ_________________________
���𰸡�b ��(Al) ���� CH4-8e-+10OH-=![]() +7H2O �����缫��2H++2e��=H2�����ٽ�ˮ���룬c(OH��)��c(H+)���Լ��� ��С Cu2++2e��=Cu
+7H2O �����缫��2H++2e��=H2�����ٽ�ˮ���룬c(OH��)��c(H+)���Լ��� ��С Cu2++2e��=Cu
��������
(1)�������˳�����Al����ǰ��Ľ����õ�ⷨұ����
(2)�������ɫ��������ʢ��ʳ��ˮ������������һ��ʱ����ɸ�ԭ�������ķ�ӦΪ����������������ԭ�����γ���ԭ��أ���֪����Ϊ������Ӧ�����������Ӧ��
(3)�ټ�װ��Ϊȼ�ϵ�أ�ȼ�ϵ����ͨ��ȼ�ϵ�һ��Ϊ������ͨ��������һ��Ϊ�������ݴ˷�����д�缫��Ӧ��
�����缫��������ˮ����������ӵõ���������������������Ũ�������ʹ��Һ�Լ��ԣ�
�۴�ͭ�е�����пҲ���������ŵ�����п���ӣ��������ŷ�Ӧ�Ľ��У�����ͭ��ҺŨ�Ƚ���С����ͭ�缫Ϊ������������ԭ��Ӧ��
(1) a.��Cu2SΪԭ�ϣ����û���ͭ����Ӧԭ��Ϊ2Cu2S+3O2![]() 2Cu2O+2SO2��2Cu2O+Cu2S
2Cu2O+2SO2��2Cu2O+Cu2S![]() 6Cu+SO2����a���������⣻
6Cu+SO2����a���������⣻
b����ҵ�������NaClұ��Na����Ӧԭ��Ϊ2NaCl�����ڣ�![]() 2Na+Cl2����b�������⣻
2Na+Cl2����b�������⣻
c����ҵ����Fe2O3Ϊԭ�ϣ����Ȼ�ԭ��ұ��������Ӧԭ��Ϊ3CO+Fe2O3![]() 2Fe+3CO2��c���������⣻
2Fe+3CO2��c���������⣻
d����HgSΪԭ��ұ��Hg��ԭ��ΪHgS+O2![]() Hg+SO2��d���������⣻
Hg+SO2��d���������⣻
��ѡb��
(2)�������⣬�������ɫ��������ʢ��ʳ��ˮ������������һ��ʱ����ɸ�ԭ�������ķ�ӦΪ��������ԭ������������ԭ�����γ���ԭ��أ���֪����Ϊ������Ӧ�����������Ӧ��������Ϊ������Ӧ�����ԭ��Ӧ��ʳ��ˮ��Ϊ�������Һ����ԭ��صĸ�����Ӧ��Ϊ����(Al)��
(3)��ȼ�ϵ�ع���ʱ��ȼ�Ϸ���������Ӧ��Ϊ������Ӧ��ڼ��������ʱ������ת��Ϊ̼������ʸ�����ӦʽΪCH4-8e-+10OH-=![]() +7H2O��
+7H2O��
�ڽ��ͼʾ��֪�����缫�����������缫�ĵ缫��ӦʽΪ2H++2e-=H2�����ٽ�ˮ���룬ʹ����������Һ��c(OH-)��c(H+)����Һ�Լ��ԣ������缫���������̪��죻
�۱�װ��Ϊ��ͭ�ľ���װ�ã����ڴ�ͭ�е�����пҲ���������ŵ�����п���ӣ��������ŷ�Ӧ�Ľ��У�����ͭ��ҺŨ�Ƚ���С����ͭ�缫Ϊ��������ͭ�Ϸ�����ԭ��Ӧ��ͭ���ӵõ�������ͭ���缫��ӦʽΪCu2++2e-=Cu��

 Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д� ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�����Ŀ���±���Ԫ�����ڱ���һ���֣�����A��H��Ԫ�����ڱ��е�λ�ã���Ԫ�ط��Ż�ѧʽ�ش��������⣺
�� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
1 | A | |||||||
2 | D | E | G | |||||
3 | B | C | F | H |
��1���ϱ��У�Ԫ�ؽ�������ǿ����________(��Ԫ�ط���)��
��2��д��D��ԭ�ӽṹʾ��ͼ��__________
��3��B��C��ԭ�Ӱ뾶�Ƚϣ� ________C (����������������)��G��H�ķǽ����ԱȽϣ�G ________ H(����������������)��
��4��A��H�γɻ�����ĵ���ʽ��_______
��5��д��B������������Ӧˮ������H����̬�⻯���ˮ��Һ��Ӧ�����ӷ���ʽ��______��
����Ŀ����ҵ���Ƚ�úת��ΪCO��������CO��ˮ������Ӧ��H2ʱ����������ƽ�⣺CO(g)��H2O(g)CO2(g)��H2(g)��
(1)ƽ�ⳣ���ı���ʽK=_________________________
(2)��1L�����ܱ������г���CO��H2O(g)��ij�¶�ʱ��ò����������±���
t/min | 0 | 1 | 2 | 3 | 4 |
n(H2O)/mol | 1.20 | 1.04 | 0.90 | 0.70 | 0.70 |
n(CO)/mol | 0.80 | 0.64 | 0.50 | 0.30 | 0.30 |
��ӷ�Ӧ��ʼ��2minʱ����H2��ʾ�ķ�Ӧ����Ϊ__________�����¶��·�Ӧ��ƽ�ⳣ��K��________(С�������2λ��Ч����)��
(3)��֪�÷�Ӧ�ڲ�ͬ���¶��µ�ƽ�ⳣ����ֵ�ֱ�Ϊ
t/�� | 700 | 800 | 830 | 1000 | 1200 |
K | 1.67 | 1.19 | 1.00 | 0.60 | 0.38 |
�ٸ��ݱ��е������жϣ��÷�ӦΪ________(��������������������)��Ӧ��
��800������2L�����ܱ������г���1molCO(g)��1molH2O(g)��2molCO2(g)��2molH2(g)����ʱv��_______v�� (����>����<����������)��
����Ŀ���ظ���أ�K2Cr2O7)�Ǹ��л�ѧ����������������ҵ���Ը�����Ϊԭ���ü����������Ʊ�����������ͨ������Cr2O3��FeO��Al2O3��SiO2�ȡ�

��֪����NaFeO2��ˮǿ��ˮ����2CrO42-(��ɫ) + 2H+![]() Cr2O72-(��ɫ)+H2O
Cr2O72-(��ɫ)+H2O
��ش��������⣺
(1)����ʯ�����Ŀ����________����������ʱCr2O3������Ӧ�Ļ�ѧ����ʽΪ__________��
(2)����1���к��ɫ���ʣ�д�����ɸ����ʷ�Ӧ�����ӷ���ʽ________������2����Ҫ�ɷ���A1(OH)3��______________��
(3)�ü�Ҫ������˵��Na2Cr2O7��Һ�м���KC1���壬��������K2Cr2O7��ԭ��_______��
(4)25��Cʱ���Է�Ӧ2CrO42-(��ɫ)+2H+![]() Cr2O72- (��ɫ)+H2O��ȡNa2CrO4��Һ����ʵ�飬��ò���ʵ���������£�
Cr2O72- (��ɫ)+H2O��ȡNa2CrO4��Һ����ʵ�飬��ò���ʵ���������£�
ʱ��/ (S) | 0 | 0.01 | 0.02 | 003 | 0.04 |
(CrO42-)/ (mol��L-1) | 0.20 | 1.6��10-2 | 1.2��10-2 | 1.0��10-2 | |
(Cr2O72-)/ (mol��L-1) | 0 | 9.2��l0-2 | 9.4��10-2 | 9.5��10-2 |
��Ӧ�ﵽƽ��ʱ����Һ��pH=l���÷�Ӧƽ�ⳣ��KΪ______��
�������й�˵����ȷ��_____________��
a.������NaHCO3���壬��ʹ��Һ�ij�ɫ����
b.0.03 sʱv(CrO42-)(��)=2v(Cr2O72-)(��)
c.��Һ��c(CrO42-):c(Cr2O72-)=2 : 1ʱ�÷�Ӧ����ƽ��״̬
d.��Ӧ�ﵽƽ��ʱCrO42-��ת����Ϊ95%