��Ŀ����
����Ŀ��Ԫ�����ڱ��еڢ�A��Ԫ�صĵ��ʼ��仯�������;�㷺��
��1������Ԫ��ͬ��Ķ�����Ԫ�ص�ԭ�ӽṹʾ��ͼΪ___��
��2������Ϊ�ȡ��塢��Ԫ�طǽ�����(ԭ�ӵõ�������)�ݱ���ɵ��ж�������___(��������ĸ���)��
a��Cl2��Br2��I2���۵� b��Cl2��Br2��I2��������
c��HCl��HBr��HI�����ȶ��� d��HCl��HBr��HI������
��3����ҵ�ϣ�ͨ������ת�����Ƶ�KClO3���壺
NaCl��Һ![]() NaClO3��Һ
NaClO3��Һ![]() KClO3����
KClO3����
����ɢ��з�Ӧ���ܻ�ѧ����ʽ��_NaCl��________H2O=________NaClO3��__________��
�ڢ���ת���Ļ�����Ӧ������___��
���𰸡�![]() bc 1 3 1 3 H2�� ���ֽⷴӦ
bc 1 3 1 3 H2�� ���ֽⷴӦ
��������
��1������Ԫ��ͬ��Ķ�����Ԫ����FԪ�ء�
��2��±��Ԫ��������������ȣ�������ԭ����������ԭ�Ӱ뾶����ԭ�Ӻ˶�����������������С���ǽ����Լ�����Ԫ�صķǽ�����Խǿ���䵥�ʵ�������Խǿ����̬�⻯����ȶ���Խǿ��
��3���ٵ��NaClˮ��Һ����������NaClO3����������������
������NaClO3��KCl����KClO3��NaCl��
��1������Ԫ��ͬ��Ķ�����Ԫ����FԪ�أ�ԭ�ӽṹʾ��ͼΪ![]() ��
��
��2��±��Ԫ��������������ȣ�������ԭ����������ԭ�Ӱ뾶����ԭ�Ӻ˶�����������������С���ǽ����Լ�����Ԫ�صķǽ�����Խǿ���䵥�ʵ�������Խǿ����̬�⻯����ȶ���Խǿ������Ϊ�ȡ��塢��Ԫ�طǽ�����(ԭ�ӵõ�������)�ݱ���ɵ��ж�������Cl2��Br2��I2�������Ժ�HCl��HBr��HI�����ȶ��ԣ����⻯����۷е㡢�⻯�������ǿ�����أ���ѡbc��
��3���ٵ��NaClˮ��Һ����������NaClO3�������������������ݵ�ʧ�����غ㣬���з�Ӧ���ܻ�ѧ����ʽ��NaCl��3H2O=NaClO3��3H2����
�ڸ������⣬����NaClO3��KCl����KClO3��NaCl�����ֻ�����������ɷ������������ֻ�������ڸ��ֽⷴӦ��

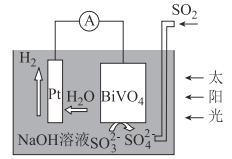
����Ŀ������ʵ�������ܴﵽʵ��Ŀ�ĵ���
ʵ��Ŀ�� | ʵ������ | |
A | �����Ҵ�����ȩ |
|
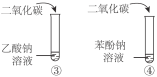
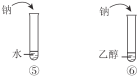
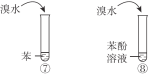
B | �Ƚ����ᡢ̼�ᡢ���ӵ����� |
|
C | ˵���������ǻ�����ԭ�ӻ��Ե�Ӱ�� | |
D | ˵��������ȡ��������ԭ�ӻ��Ե�Ӱ�� |
|
A. AB. BC. CD. D
����Ŀ����ˮ��������M�ķֽ�����Ӱ�����ؽ����о�������ͬ�¶��£�M�����ʵ���Ũ��(mol��L-1)��ʱ��(min)�仯���й�ʵ�����ݼ��±���
ʱ�� ˮ�� | 0 | 5 | 10 | 15 | 20 | 25 |
I (pH=2) | 0.40 | 0.28 | 0.19 | 0.13 | 0.10 | 0.09 |
II(pH=4) | 0.40 | 0.31 | 0.24 | 0.20 | 0.18 | 0.16 |
��(pH=4) | 0.20 | 0.15 | 0.12 | 0.09 | 0.07 | 0.05 |
IV(pH=4���� Cu2+) | 0.20 | 0.09 | 0.05 | 0.03 | 0.01 | 0 |
����˵������ȷ���ǣ� ��
A.����Cu2+���ڣ�IV��M�ķֽ����ʱ�I��
B.����������ͬʱ��ˮ������Խǿ��M�ķֽ�����Խ��
C.��0��25 min�ڣ�����M�ķֽ�ٷ��ʱ�II
D.��0��20 min�ڣ�I��M��ƽ���ֽ�����Ϊ0.015mol/(L��min)