题目内容
【题目】将铁粉和铝粉的混合物逐渐加入100mL的稀硝酸中,其产生的有关离子浓度与加入铁粉和铝粉的混合物的质量的关系如下图所示:
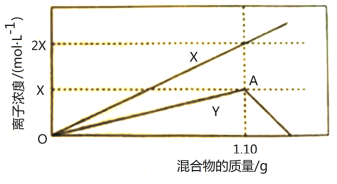
若反应过程中生成的气体为NO,溶液的体积变化忽略不计。请回答下列问题:
(1)X表示的变化离子是_______________(填离子符号)。
(2)OA段发生反应的离子方程式是___________________________________。
(3)稀硝酸溶解1.10g铁粉和铝粉后溶液还能继续溶解混合物的原因是________________
(4)该硝酸的浓度是_____________mol·L-1。
【答案】Al3+ Fe+ 4H++ NO3-== Fe 3++NO↑+2H2O 稀硝酸溶解1.10g铁粉和铝粉生成的是Fe 3+,Fe 3+还能与Al和Fe反应生成Al3+和Fe 3+ 0.9
【解析】
将铁粉和铝粉的混合物逐渐加入100mL的稀硝酸中,刚开始硝酸过量,Fe和稀硝酸反应生成Fe(NO)3,继续添加至Fe过量,发生反应Fe+2 Fe(NO)3=3 Fe(NO)2,Fe3+离子浓度降低直至完全没有;Al和稀硝酸反应生成Al(NO)3,硝酸反应完后,Al还可以和溶液中的Fe 3+和Fe 2+反应,生成Al3+,故Al3+浓度一直上升,以此解答。
(1)由分析可知,浓度一直增大的是Al3+;
(2)由分析可知,OA段发生Fe和稀硝酸反应生成Fe(NO)3的反应,离子方程式为:Fe+ 4H++ NO3-== Fe 3++NO↑+2H2O;
(3)稀硝酸溶解1.10g铁粉和铝粉生成的是Fe 3+,Fe 3+还能与Al和Fe反应生成Al3+和Fe 3+;
(4)当混合物的质量为1.10g时,稀硝酸完全反应,此时2n(Fe3+)=n(Al3+),故混合物中2n(Fe)=n(Al),设n(Fe)=xmol,n(Al)=2xmol,有56x+27×2x=1.10,解得x=0.01,则n(HNO3)=3n(Fe3+)+ 3n(Al3+)=0.09mol,该硝酸的浓度是![]() =
= ![]() =0.9mol/L。
=0.9mol/L。