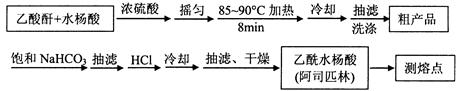
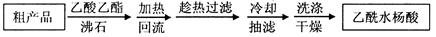��Ŀ����
��16�֣���������泥�NH2COONH4����һ�ְ�ɫ���壬�����ֽ⡣ijС��ģ���Ʊ���������泥���Ӧ���£����¶ȶԷ�Ӧ��Ӱ��Ƚ���������2NH3(g)+CO2(g) NH2COONH4(s) ��H��0
NH2COONH4(s) ��H��0
��1��������ͼIװ����ȡ��������ѡ����Լ��� ��
��2���Ʊ���������淋�װ������ͼ����ʾ����NH3��CO2ͨ�����Ȼ�̼�У����Ͻ����ϣ����ɵİ�������淋�С����������CCl4�С� ��������϶�ʱ��ֹͣ�Ʊ���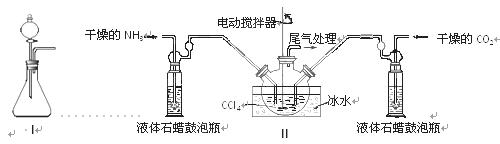
ע��CCl4��Һ��ʯ����Ϊ���Խ��ʡ�
�ٷ������ñ�ˮ��ȴ��ԭ����_________________________________________��Һ��ʯ������ƿ�������� ��
�ڴӷ�Ӧ��Ļ�����з������Ʒ��ʵ�鷽���� ����д�������ƣ���Ϊ�˵õ������Ʒ��Ӧ��ȡ�ķ�����________����дѡ����ţ���
a����ѹ���Ⱥ�� b����ѹ���Ⱥ�� c����ѹ40�����º��
��3���Ƶõİ�������刺��ܺ���̼����李�̼����е�һ�ֻ����֡�
����Ʒ��������гɷ�̽��������д���пո�
��ѡ�Լ�������ˮ��ϡHNO3��BaCl2��Һ������ʯ��ˮ��AgNO3��Һ��ϡ���ᡣ
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡ����������Ʒ���Թ��У���������ˮ�������ܽ⡣ | �õ���ɫ��Һ |
| ����2�����Թ��м��������BaCl2��Һ������ | ����Һ������ǣ�֤�������в���̼��李� |
| ����3�����Թ��м������� �� | ��֤�������к���̼����李� |
��ÿ��2�֣�
��1��Ũ��ˮ���������ƹ���Ⱥ�����
��2���ٽ����¶ȣ���߷�Ӧ��ת���ʣ����¶ȣ���ֹ��Ӧ������ɲ���ֽ⣩��
ͨ���۲����ݣ�����NH3��CO2ͨ���������ͨ���۲����ݣ�����NH3��CO2�ķ�Ӧ���ʣ�
�ڹ��� c
��3����
��0.800��80.0%����Ч����û�п��Dz��۷֣�ʵ�鲽�� Ԥ������ͽ��� ����3����������ʯ��ˮ ��Һ�����
���������������ͼIװ����û�м��ȣ�ֻ����Ũ��ˮ���������ƹ���������ƹ��巴Ӧ��ȡ������
�Ƣپ���Ϣ���÷�ӦΪ���ȷ�Ӧ�������ﰱ������泥�NH2COONH4�������ֽ⡱�����Ϊ����߷�Ӧ��� ת���ʻ��ֹ����ķֽ⣬���ñ�ˮ��ȴ������������ͨ��Һ��ʯ������ƿʱ�ܹ۲�������������NH3��CO2ͨ����������NH3��CO2�ķ�Ӧ���ʣ�
�ڸ��ݡ����ɵİ�������淋�С����������CCl4�С�˵�����������������CCl4��Һ�壩�����ù��˲����ܽ���������林ӻ�����з��������Ϊ�˷�ֹ������������ȷֽ⣬���á���ѹ40�����¡���ɲ�Ʒ��
�Ǣ��ڡ���ѡ�Լ�����ϡ���ᡢϡ�����백������李�̼����茶��ܷ�Ӧ�������壬����������֤̼����泥�ֻ�г���ʯ��ˮ��̼����立�Ӧ����̼��Ƴ�������Һ����ǣ���
�ڸ��ݢٿ�֪��Ʒ�ijɷ���NH2COONH4��NH4HCO3������������������Һ��ַ�Ӧ����BaCO3���������ʵ���Ϊn(BaCO3)=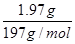 =0.01mol=n(NH4HCO3)[��̼ԭ���غ�]������Ʒ�а�������淋����ʵ�������Ϊ
=0.01mol=n(NH4HCO3)[��̼ԭ���غ�]������Ʒ�а�������淋����ʵ�������Ϊ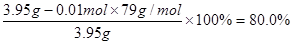 ��
��
���㣺 ���⿼�����ʵ��Ʊ����������ᴿ�����ʳɷֵ�̽������֤����Ʒ���ȵļ���ȡ�

 �����������Ż�ѧϰϵ�д�
�����������Ż�ѧϰϵ�д���18�֣�NO���������������Ѹ�ٷ�����Ӧ����ѧ�ҷ������������в��ϵز���NO������ϸ���䴫����Ϣ��NO��������Ѫ��ϵͳ������ϵͳ�Լ��������Χ��ϵͳ�ĵ��ء�
��.��1��ʵ�����ý���ͭ��ϡ������ȡNO�����ӷ���ʽΪ
_____________________________________________________��
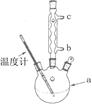
��2��NO���ж����壬ijѧ��Ϊ��ֹ��Ⱦ���÷�Һ©�����ձ�װ����һ���ġ����濪���á������ͣ��NO���巢��װ�ã���ͼ����ʾ��
��ʵ������û��ͭ˿����ֻ��Сͭ������ʹ������װ�ý���ʵ��ʱ������˿״���ϰ���ͭ���Դ���ͭ˿����ʵ�飬����˿״���ϵijɷֿ�����________(��ѡ����)��
| A���� | B���� | C���� | D������ |

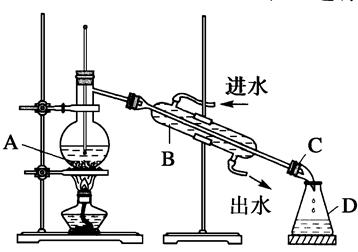
��3��Ϊ֤��ͭ˿��ϡ���ᷴӦ���ɵ�ȷʵ��NO��ijѧ���������һ����ͼ����ʾ��װ����ȡNO�����Ϳ�ʼ������U�ι��Ҷ˹۲쵽��ɫ��NO���塣
�ٳ������ܵ�������___________________________________��
���÷�Ӧֹͣ�IJ���������ԭ����________________________��
��4�������ռ�NO�����װ�ã���������________(��ѡ�����)��

��5������ʵ����12.8 g Cuȫ���ܽ⣬��Ҫͨ������________mL O2����ʹNOȫ������ˮ��
���ý���ͭ��ȡ����ͭ���ӽ�Լԭ�Ϻͷ�ֹ������Ⱦ�ĽǶȿ��ǣ�����4�ַ�������õ���________(��ס������ҡ�������������)��������___________________________________________��

�ú���A12O3��SiO2������FeO��xFe2O3�������Ʊ�A12(SO4)3��18H2O�������������£����ֲ����������ԣ�
���������м������ϡH2SO4������:
������Һ�м������KMnO4��Һ��������Һ��pHԼΪ3��
���ȣ�����������ɫ���������ã��ϲ���Һ���Ϻ�ɫ:
��������MnSO4���Ϻ�ɫ��ʧ������;
����Ũ�����ᾧ�����룬�õ���Ʒ��
��1��H2SO4�ܽ�A12O3�����ӷ���ʽ��
��2��KMnO4����Fe2+�����ӷ���ʽ����������
��3����֪�������������������pH
| | Al(OH)3 | Fe(OH)2 | Fe(OH)3 |
| ��ʼ����ʱ | 3.4 | 6.3 | 1.5 |
| ��ȫ����ʱ | 4.7 | 8.3 | 2.8 |
���ݱ������ݽ��Ͳ�����Ŀ�� ��
��4����֪��һ�������£�MnO4-����Mn2+��Ӧ����MnO2,
�����ij����м���ŨHCI�����ȣ���˵�������д���MnO2�������� ��
�ڢ��м���MnSO4��Ŀ���� ��
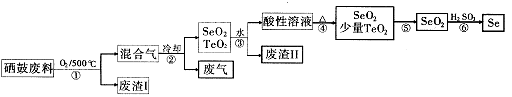
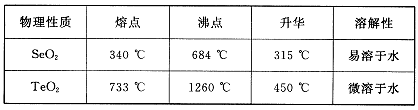


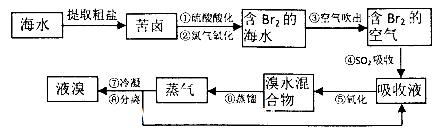
 ����������Ӧ����㷺�Ľ��ȡ���ʹ�Ϳ���ҩ������ˮ���������ֽ⣬�ֽ��¶�Ϊ128��135�档ijѧϰС����ʵ������ˮ���ᣨ���ǻ������ᣩ�������[(CH3CO)2O]Ϊ��Ҫԭ�Ϻϳɰ�˾ƥ�֣��Ʊ����������������£�
����������Ӧ����㷺�Ľ��ȡ���ʹ�Ϳ���ҩ������ˮ���������ֽ⣬�ֽ��¶�Ϊ128��135�档ijѧϰС����ʵ������ˮ���ᣨ���ǻ������ᣩ�������[(CH3CO)2O]Ϊ��Ҫԭ�Ϻϳɰ�˾ƥ�֣��Ʊ����������������£�