��Ŀ����
��12�֣�����ͭ��������������Ӧ�ù㷺��ij�������ú��������ķ�ͭ��Ϊԭ�������������������£�
��1��д������ʱͭ��ϡ���ᡢϡ���ᷴӦ��������ͭ�Ļ�ѧ����ʽ�� ��
��2��ȡ��������Ϊ��ȷ��Fe3+�Ƿ��������ļ��鷽���� ��
��3������c�� ��
��4������a���Ա�ѭ�����ã��û�ѧ����ʽ��ʾ����a��ѭ�����õ�ԭ��Ϊ��
2NO+O2 =2NO2�� ��
��5��һ���¶��£�����ͭ���ȷֽ�����CuO��SO2���塢SO3�����O2���壬д������ͭ���ȷֽ�Ļ�ѧ����ʽ�� ��
ijͬѧ���������ͼ��ʾ��ʵ��װ�÷ֱ�ⶨ���ɵ�SO2���塢SO3�����������O2����������������в�����֮������˵�����ɣ� ��
��12�֡�
��1��3Cu + 2HNO3 + 3H2SO4 =3CuSO4 + 2NO��+ 4H2O��2�֣�
��2���������еμ�KSCN��Һ������Һ�Ժ�ɫ����Fe3+δ����������Fe3+������2�֣�
��3��Fe(OH)3��2�֣�
��4��3NO2+H2O=2HNO3+NO��2�֣�
��5��3CuSO4 3CuO + SO3��+ 2SO2��+ O2���������𰸾��ɣ���2�֣�
3CuO + SO3��+ 2SO2��+ O2���������𰸾��ɣ���2�֣�
NaHSO3��������SO3��ͻ����ղ���O2�������𰸾��ɣ���2�֣�
���������������1������������ԭ��Ӧ���ۣ�ͭ��ϡ���ᡢϡ����Ļ��Һ��Ӧ��������ͭ��һ��������ˮ����ѧ����ʽΪ3Cu + 2HNO3 + 3H2SO4 =3CuSO4 + 2NO��+ 4H2O��
��2��Fe3+�ļ��鷽���ǣ��������еμ�KSCN��Һ������Һ�Ժ�ɫ����Fe3+δ����������Fe3+������
��3����ͭ���к��������������Ե���pHĿ����ʹ�����ӳ�����������c��Fe(OH)3��
��4������a��NO��NO��������Ӧ���ɶ���������������������ˮ�ֵ������NO����ѧ����ʽΪ3NO2+H2O=2HNO3+NO��
��5������ͭ���ȷֽ�����CuO��SO2���塢SO3�����O2���壬��ƽ����ʽ��3CuSO4 3CuO + SO3��+ 2SO2��+ O2��������NaHSO3��Һ�����ն���������������������Ӧ�����ɶ��������¶���������������
3CuO + SO3��+ 2SO2��+ O2��������NaHSO3��Һ�����ն���������������������Ӧ�����ɶ��������¶���������������
���㣺���黯ѧ����ʽ���ж�����д�����Ӽ��飬�����жϣ�װ�õ��ж�

ʵ�����Ʊ�1��2-��������ķ�Ӧԭ�����£�
CH3CH2OH CH2=CH2+H2O
CH2=CH2+H2O
CH2=CH2+Br2��BrCH2CH2Br
���ܴ��ڵ���Ҫ����Ӧ�У��Ҵ���Ũ����Ĵ�������140����ˮ�������ѣ�Ũ������Ҵ�����ΪCO2�ȡ�
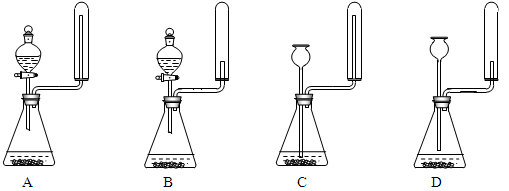
������������������Ҵ��Ʊ�1��2-���������װ������ͼ��ʾ��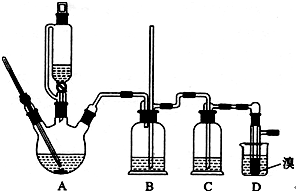
����������:
| | �Ҵ� | 1,2-�������� | ���� |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶ�/g��cm3 | 0��79 | 2��2 | 0��71 |
| �е�/oC | 78��5 | 132 | 34��6 |
| �۵�/oC | -130 | 9 | -116 |
��1��Aװ���Ϸ�ʹ�õ�Һ©�����ŵ��ǣ�_________________________���������һ��ʱ��������ǼӴ�Ƭ��Ӧ�ò�ȡ����ȷ������_____________________(����ȷ�𰸱��)��
A���������� B����ȴ�� C�����貹�� D����������
��2��Bװ�õ�������_____________________________________��
��3����װ��C��Ӧ����________(����ȷѡ��ǰ����ĸ)����Ŀ����______________��
a��ˮ b��Ũ���� c������������Һ d������̼��������Һ
��4���жϸ��Ʊ���Ӧ�Ѿ��������������____________________________��
��5��Dװ�þ�֧�Թ���������ˮ����Һ��(�ٶ�������ͬ)���������ŵ�________________��
��6����Ӧ������Ӧ����ˮ��ȴװ��D������ҪĿ����_____________�����ֲ��ܹ�����ȴ(���ñ�ˮ)����ԭ����_________________________��
��16�֣���������泥�NH2COONH4����һ�ְ�ɫ���壬�����ֽ⡣ijС��ģ���Ʊ���������泥���Ӧ���£����¶ȶԷ�Ӧ��Ӱ��Ƚ���������2NH3(g)+CO2(g)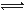 NH2COONH4(s) ��H��0
NH2COONH4(s) ��H��0
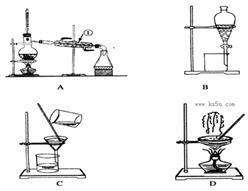
��1��������ͼIװ����ȡ��������ѡ����Լ��� ��
��2���Ʊ���������淋�װ������ͼ����ʾ����NH3��CO2ͨ�����Ȼ�̼�У����Ͻ����ϣ����ɵİ�������淋�С����������CCl4�С� ��������϶�ʱ��ֹͣ�Ʊ���
ע��CCl4��Һ��ʯ����Ϊ���Խ��ʡ�
�ٷ������ñ�ˮ��ȴ��ԭ����_________________________________________��Һ��ʯ������ƿ�������� ��
�ڴӷ�Ӧ��Ļ�����з������Ʒ��ʵ�鷽���� ����д�������ƣ���Ϊ�˵õ������Ʒ��Ӧ��ȡ�ķ�����________����дѡ����ţ���
a����ѹ���Ⱥ�� b����ѹ���Ⱥ�� c����ѹ40�����º��
��3���Ƶõİ�������刺��ܺ���̼����李�̼����е�һ�ֻ����֡�
����Ʒ��������гɷ�̽��������д���пո�
��ѡ�Լ�������ˮ��ϡHNO3��BaCl2��Һ������ʯ��ˮ��AgNO3��Һ��ϡ���ᡣ
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡ����������Ʒ���Թ��У���������ˮ�������ܽ⡣ | �õ���ɫ��Һ |
| ����2�����Թ��м��������BaCl2��Һ������ | ����Һ������ǣ�֤�������в���̼��李� |
| ����3�����Թ��м������� �� | ��֤�������к���̼����李� |
ʵ��������������ˮ���������Ҵ��Ʊ�1��2�����������װ������ͼ��ʾ��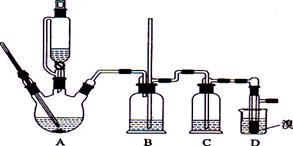
�й������б����£�
| | �Ҵ� | 1,2-�������� | ���� |
| ״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶȣ�g �� cm-3 | 0.79 | 2.2 | 0.71 |
| �е㣯�� | 78.5 | 132 | 34.6 |
| �۵㣯�� | -l30 | 9 | -1l6 |

�ش��������⣺
��1����ƿD�з�������Ҫ�ķ�Ӧ����ʽ ��
��2����ȫƿB���Է�����,�����Լ��ʵ�����ʱ�Թ�D�Ƿ�����������д����������ʱƿB�е����� ��
��3����װ��C��Ӧ���� (����ĸ) ����Ŀ����_______________��
a��ˮ b��Ũ���� c������������Һ
��4����������������δ��Ӧ��Br2������� ϴ�ӳ�ȥ��������ĸ��
a��ˮ b������������Һ c���⻯����Һ d���Ҵ�
��ȡ�������Ҫ���������� ��
��5�������������������������ѣ����� �ķ�����ȥ��
��6����Ӧ������Ӧ����ˮ��ȴװ��D�����ֲ��ܹ�����ȴ�����ñ�ˮ������ԭ���� ��
��7���жϸ��Ƹ���Ӧ�Ѿ�������������� ��
�����л�����ϩ������ȥ��ϩ�õ������ļ��飬�������ͨ��ʢ��������Щ�Լ���ϴ��ƿ�� ��
| A������ʯ��ˮ��ŨH2SO4 | B������KMnO4��ŨH2SO4 |
| C����ˮ��ŨH2SO4 | D��ŨH2SO4����ˮ |



 3NH3 + 8AlO2��
3NH3 + 8AlO2��
