��Ŀ����
��1����4�֣���¯���������з�������Ҫ��ӦΪ��1/3Fe2O3(s)+CO(g)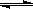 2/3Fe (s)+CO2(g)����֪�÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�ⳣ�����¡���
2/3Fe (s)+CO2(g)����֪�÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�ⳣ�����¡���

�ٸ÷�Ӧ�ġ�H_____0(�>������<����=��)��
����һ���ݻ�Ϊ10L���ܱ������У�1000��ʱ����Fe��Fe2O3��CO��CO2��1.0 mol����Ӧ����l0 min��ﵽƽ�⡣��CO��ƽ��ת����= ________��
��2����3�֣������£�HR���ᣩ��ҺpH=3��MOH�������ҺpH=11�����ߵ������Ϻ���Һ�Լ��ԡ�������Һ�бض���һ�������ܷ���ˮ�⣬��ˮ�ⷴӦ�����ӷ���ʽ
___________________________________________��
��3����3�֣�25��ʱ��pH=0��HCl��0.1mol/L��HCl��0.01mol/L��NaOH��pH=14��NaOH������Һ����ˮ���������c��H+��֮��Ϊ ��
��4����3�֣���֪25��ʱ�����볣��Ka(HF)��3.6��10��4����0.1 mol��L��1 HF��Һ��c(H��)�� mol��L��1��
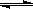 2/3Fe (s)+CO2(g)����֪�÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�ⳣ�����¡���
2/3Fe (s)+CO2(g)����֪�÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�ⳣ�����¡���
�ٸ÷�Ӧ�ġ�H_____0(�>������<����=��)��
����һ���ݻ�Ϊ10L���ܱ������У�1000��ʱ����Fe��Fe2O3��CO��CO2��1.0 mol����Ӧ����l0 min��ﵽƽ�⡣��CO��ƽ��ת����= ________��
��2����3�֣������£�HR���ᣩ��ҺpH=3��MOH�������ҺpH=11�����ߵ������Ϻ���Һ�Լ��ԡ�������Һ�бض���һ�������ܷ���ˮ�⣬��ˮ�ⷴӦ�����ӷ���ʽ
___________________________________________��
��3����3�֣�25��ʱ��pH=0��HCl��0.1mol/L��HCl��0.01mol/L��NaOH��pH=14��NaOH������Һ����ˮ���������c��H+��֮��Ϊ ��
��4����3�֣���֪25��ʱ�����볣��Ka(HF)��3.6��10��4����0.1 mol��L��1 HF��Һ��c(H��)�� mol��L��1��
��16�֣�
��1����4�֣�< 60%
��2����3�֣�M++H2O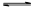 MOH+H+
MOH+H+
��3����3�֣�1��10��100��1
��4����3�֣�6��10��3
��5����3�֣�7
��1����4�֣�< 60%
��2����3�֣�M++H2O
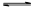 MOH+H+
MOH+H+��3����3�֣�1��10��100��1
��4����3�֣�6��10��3
��5����3�֣�7
�������������1�����ɱ������ݿ�֪���¶�Խ��ƽ�ⳣ��ԽС���������¶�ƽ�����淴Ӧ�ƶ�������ӦΪ���ȷ�Ӧ������H��0������ƽ��ʱCO�����ʵ����仯Ϊnmol����
1/3Fe2O3��s��+CO��g��
 2/3Fe��s��+CO2��g��
2/3Fe��s��+CO2��g����ʼ��mol����1 1
�仯��mol����n n
ƽ�⣨mol����1-n 1+n
����(1+n)/(1-n) =4�����n=0.6��
����n��CO��=0.6mol��1mol��100%=60%��
��2����������Ĺ��ɡ�˭��˭ˮ�⣬˭ǿ��˭�ԡ���MR������ǿ���Σ���ˮ�ⷴӦ�����ӷ���ʽΪM++H2O
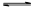 MOH+H+��
MOH+H+����3��������Һ����ˮ���������c��H+���ֱ�Ϊ��10-14��10-13��10-12��10-14��֮��Ϊ1��10��100��1��
��4�����볣��Ka(HF)��c(H��)��c(F-)��c(HF) =3.6��10��4��c(HF)=" 0.1" mol��L��1��c(H��)=c(F-)����c(H��)��6��10��3��
��5����3�֣����¶�t ��ʱ��pH=3��ijˮ��Һ��c(OH��)=10��8 mol��L���ڴ��¶���5��10��5mol/LBa��OH��2��Һ��pH= ��
��5�����¶�t ��ʱ�����ӻ�����K= c(OH��)��c(H+)=10-3 (mol��L)��10��8 (mol��L) =10��11 (mol��L)2��5��10��5mol/LBa��OH��2��Һ�У�c(OH��)=10��4 mol��L������K= c(OH��)��c(H+)����c(H��)= 10��7 mol��L��pH=7��
���������⿼������ۺϵ�֪ʶ����Ŀ�Ѷ��У�ע�����֪ʶ��ѧϰ�����ռ�Ӧ�á�

��ϰ��ϵ�д�
 Сѧ�̲���ȫ���ϵ�д�
Сѧ�̲���ȫ���ϵ�д�
�����Ŀ
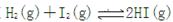 �ﵽƽ��״̬ʱ�ı�־��
�ﵽƽ��״̬ʱ�ı�־��
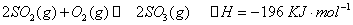 ʱ����2L�̶��ݻ����ܱ������г���2mol
ʱ����2L�̶��ݻ����ܱ������г���2mol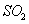 ��2mol
��2mol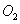 ��10���Ӻ�ﵽƽ�⣬
��10���Ӻ�ﵽƽ�⣬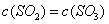
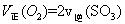 )
)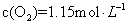 ������ͼ�ϻ���15-25����
������ͼ�ϻ���15-25����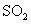 ��Ũ�ȱ仯���ߡ�
��Ũ�ȱ仯���ߡ�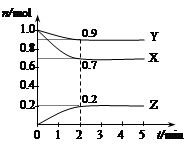
 2Z(g)
2Z(g)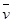 (X)��0.075 mol ��L-1�� min-1
(X)��0.075 mol ��L-1�� min-1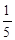
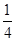
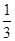
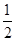

 2C(g)��ƽ�⣬���ʼA��B�����ʵ����ֱ�ΪXmol��Ymol����Ҫʹƽ��ʱ��Ӧ�������ʵ����������������ʵ�����ȣ���X/YӦ�������
2C(g)��ƽ�⣬���ʼA��B�����ʵ����ֱ�ΪXmol��Ymol����Ҫʹƽ��ʱ��Ӧ�������ʵ����������������ʵ�����ȣ���X/YӦ�������