��Ŀ����
����Ŀ����Ҫ����գ�
��1��ij����A���������ܶ�����ͬ״���������ܶȵ�64�������ⶨ��֪A�����й�����6������
����A����ϩ���������ӳɵIJ����A������Ϊ��______��
����A��Ȳ���������ӳɵIJ����A���ṹ��ʽΪ��____��
��2��ij��B 0.1 mol �������г��ȼ�պ�������7.2gH2O���Իش��������⣺
������B����ʹ��ˮ��ɫ������һ��������������������ȡ����Ӧ����һ��ȡ���������֣�����B�Ľṹ��ʽΪ_____��
������B����ʹ��ˮ��ɫ������ʹ���Ը��������Һ��ɫ������B�Ŀ��ܵĽṹ��ʽΪ_______
������B��ʹ��ˮ��ɫ���ڴ��������£���H2�ӳɣ���ӳɲ��ᆳ�ⶨ�����к���3����������B���ܵĽṹ��ʽ_____��
��3��ij��C����Է�������Ϊ84���ش��������⣺
������������C�����������ϣ��������ʵ���һ�������ȼ������������������ȵ���(�����)___��
a��C7H12O2 b��C6H14 c��C6H14O d��C7H14O3
������CΪ��������HBr�ӳɺ�ֻ�ܵõ�һ�ֲ���Ҹ�����һ�ȴ���ֻ��һ�֡�C�Ľṹ��ʽΪ________��������_____����һ���������ܷ����Ӿ۷�Ӧ��д���÷�Ӧ�Ļ�ѧ����ʽ_____ ��
���𰸡�2��2��4��4-�ļ����� (CH3)3CC(CH3)2CH2CH3 CH3CH2CH3 ![]() CH2=C(CH3)2 b (CH3)2C=C(CH3)2 2��3������-2-��ϩ
CH2=C(CH3)2 b (CH3)2C=C(CH3)2 2��3������-2-��ϩ 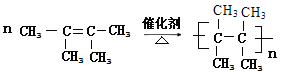
��������
��1������ܶ�֮�ȵ�Ħ������֮�ȣ�����A�����ܶ�����ͬ״���������ܶȵ�64������M=128g/mol������ʽ����CnH2n+2�������ʽΪC9H20����֪A�����й�����6��������������5��̼ԭ�ӣ�
��2��ij��B�ķ���ʽΪCxHy��ȼ�յķ���ʽΪCxHy+��x+y/4��O2![]() xCO2+y/2H2O����1��y/2=0.1��0.4��y=8��B�ķ���ʽΪCxH8��
xCO2+y/2H2O����1��y/2=0.1��0.4��y=8��B�ķ���ʽΪCxH8��
��3��ij��C����Է�������Ϊ84�������ʽΪC6H12��
��1��������ܶ�֮�ȵ�Ħ������֮�ȣ�����A�����ܶ�����ͬ״���������ܶȵ�64������M=128g/mol������ʽ����CnH2n+2�������ʽΪC9H20����֪A�����й�����6��������������5��̼ԭ�ӣ�ϩ���������ӳɣ�������������̼ԭ���ϸ�����һ����ԭ�ӣ�A����ϩ���������ӳɵIJ����4������2��2��4��4λ���ϣ�����Ϊ��2��2��4��4-�ļ����飻
��A��Ȳ���������ӳɵIJ������������̼ԭ����ͬʱ��������2����ԭ�ӣ��ṹ��ʽΪ��(CH3)3CC(CH3)2CH2CH3��
��2��ij��B�ķ���ʽΪCxHy��ȼ�յķ���ʽΪCxHy+��x+y/4��O2![]() xCO2+y/2H2O����1��y/2=0.1��0.4��y=8��B�ķ���ʽΪCxH8��
xCO2+y/2H2O����1��y/2=0.1��0.4��y=8��B�ķ���ʽΪCxH8��
����B����ʹ��ˮ��ɫ����һ��ȡ���������֣���BΪ���飬��ṹ��ʽΪCH3CH2CH3��
������B����ʹ��ˮ��ɫ������ʹ���Ը��������Һ��ɫ����BΪ����ͬϵ�����ʽΪC7H8���ṹ��ʽΪ��![]() ��
��
������B��ʹ��ˮ��ɫ���ڴ��������£���H2�ӳɣ���ӳɲ��ᆳ�ⶨ�����к���3����������ʽΪC4H8������̼̼˫�����ṹ��ʽΪ��CH2=C(CH3)2��
��3��ij��C����Է�������Ϊ84�������ʽΪC6H12��
�ٸ�������C�����������ϣ��������ʵ���һ�������ȼ����������������ȣ����л�����C�ڷ�����������1�������ɸ���CO2������H2O����
a��C7H12O2��C����������1����CO2���� ����C�����������ϣ��������ʵ���һ�������ȼ�����������������䣬���������⣻
b��C6H14��C����������2��H������C�����������ϣ��������ʵ���һ�������ȼ������������������ȣ������������
c��C6H14O��C����������1����H2O��������C�����������ϣ��������ʵ���һ�������ȼ�����������������䣬���������⣻
d��C7H14O3��C����������1����CH2O3���� ����C�����������ϣ��������ʵ���һ�������ȼ�����������������䣬���������⣻
��Ϊb��
������CΪ��������HBr�ӳɺ�ֻ�ܵõ�һ�ֲ����C����̼̼˫���Գƣ�������һ�ȴ���ֻ��һ�֣�C�Ľṹ��ʽΪ(CH3)2C=C(CH3)2������Ϊ2��3������-2-��ϩ���䷢���Ӿ۷�Ӧ�ķ���ʽΪ�� ��
��

����Ŀ������ϩ��![]() ���������������ϵ���Ҫ���壬��ͨ���ұ��������Ƶã�
���������������ϵ���Ҫ���壬��ͨ���ұ��������Ƶã�![]()
![]()
![]() ��H2(g)
��H2(g)
��1����֪��
��ѧ�� | C-H | C-C | C=C | H-H |
����/kJ/mol | 412 | 348 | 612 | 436 |
����������Ӧ����1mol��������ЧӦ___�������Ŷ���kJ��
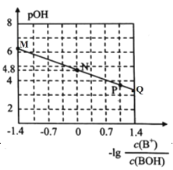
��2����ҵ�ϣ�ͨ�����ұ���EB�������в���N2��ԭ�������ұ���N2�����ʵ���֮��Ϊ1�U10��N2�����뷴Ӧ�������Ʒ�Ӧ�¶�600�棬��������ϵ��ѹΪ0.1Mpa����������½��з�Ӧ���ڲ�ͬ��Ӧ�¶��£��ұ���ƽ��ת���ʺ�ij���������±���ϩ��ѡ���ԣ�ָ����H2����IJ����б���ϩ�����ʵ���������ʾ��ͼ��
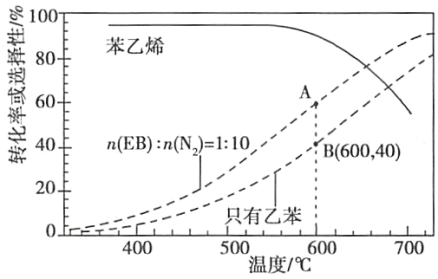
��A��B�����Ӧ������Ӧ���ʽϴ����___��
�ڿ��Ʒ�Ӧ�¶�Ϊ600���������___��
����Ŀ������ͼ��ʾ��װ�ý���ʵ�飬����![]() ��
��![]() ��
��![]() �зֱ�ʢ���Լ�1��2��3�������ܴﵽʵ��Ŀ���ǣ� ��
�зֱ�ʢ���Լ�1��2��3�������ܴﵽʵ��Ŀ���ǣ� ��
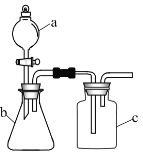
ѡ�� | �Լ�1 | �Լ�2 | �Լ�3 | ʵ��Ŀ�� |
A | Ũ���� |
| ����ʳ��ˮ | �Ʊ� |
B | Ũ���� | �Ҵ� | ��ˮ | ��֤��ϩ��ʹ��ˮ��ɫ |
C | ���� |
| Ʒ����Һ | ֤�� |
D | ϡ���� | ��Һ | ����ʯ��ˮ | ��֤ |
A.AB.BC.CD.D
����Ŀ���⼰�仯����������������ͿƼ��ȷ��涼������Ҫ��Ӧ�á��ش��������⣺
(1)��֪����![]()
��![]()
��![]()
��![]() ��
��![]() ��Ӧ����
��Ӧ����![]() ���Ȼ�ѧ����ʽΪ________________________________��
���Ȼ�ѧ����ʽΪ________________________________��
(2)��Ӧ��![]() ����716Kʱ���ݻ���Ϊ1L��A��B�����ܱ������У���ʼʱA�����г���1mol HI��B�����г���
����716Kʱ���ݻ���Ϊ1L��A��B�����ܱ������У���ʼʱA�����г���1mol HI��B�����г���![]() ��
��![]() ��0.5mol�����������е⻯������ʵ���
��0.5mol�����������е⻯������ʵ���![]() �뷴Ӧʱ��
�뷴Ӧʱ��![]() �Ĺ�ϵ���±���
�Ĺ�ϵ���±���
| 0 | 20 | 40 | 60 | 80 | 120 |
A���� | 1 | 0.91 | 0.85 | 0.81 | 0.795 | 0.784 |
B���� | 0 | 0.6 | 0.73 | 0.77 | 0.78 | 0.784 |
��120minʱ�����ж�A��B������Ӧ����ƽ��״̬��������________________��716Kʱ���÷�Ӧ��ƽ�ⳣ��K=_________________��ֻ�м���ʽ���ɣ���
��������Ӧ�У�����Ӧ����Ϊ![]() ���淴Ӧ����Ϊ
���淴Ӧ����Ϊ![]() ������
������![]() ��
��![]() Ϊ���ʳ�������
Ϊ���ʳ�������![]() ________________����K��
________________����K��![]() ���ʾ����
���ʾ����
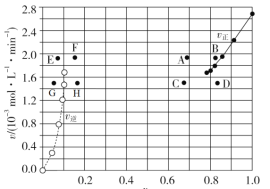
����A������ʵ�����ݼ���õ�![]() ��
��![]() �Ĺ�ϵ������ͼ��ʾ��
�Ĺ�ϵ������ͼ��ʾ��![]() Ϊ���ʵ����������������͵�ijһ�¶�ʱ����Ӧ���´ﵽƽ�⣬
Ϊ���ʵ����������������͵�ijһ�¶�ʱ����Ӧ���´ﵽƽ�⣬![]() ��
��![]() ���Ӧ�ĵ���ֱܷ�Ϊ____��______������ĸ��������A�����м����������ﵽƽ��ʱ�����Ӧ��ĺ�����ֵ______���������С�����䡱����ͬ����������ֵ________��
���Ӧ�ĵ���ֱܷ�Ϊ____��______������ĸ��������A�����м����������ﵽƽ��ʱ�����Ӧ��ĺ�����ֵ______���������С�����䡱����ͬ����������ֵ________��
����Ŀ����������(POCl3)��һ�ֹ�ҵ����ԭ�ϣ���������ȡ�л���ũҩ����Ч�ǰ�ҩ��ȣ���������Ⱦ���м��塢�л��ϳɵ��Ȼ����ʹ�������ȼ���ȡ�����O2��PCl3Ϊԭ�Ͽ��Ʊ���������,���Ʊ�װ����ͼ��ʾ(�г�װ����ȥ)��
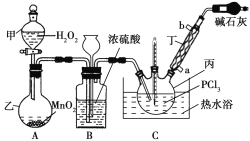
��֪PCl3�������������������
�۵�/�� | �е�/�� | ����������ѧ���� | |
PCl3 | -112.0 | 76.0 | PCl3��POCl3���ܣ���Ϊ��ɫҺ�壬��ˮ������ˮ�⣬�������ֽⷴӦ�����ĺ������HCl |
POCl3 | 1.25 | 106.0 |
��1��װ��A�еķ�Һ©���ܷ��ó���©�����棿�����жϲ�����ԭ��_______
��2��װ��B��������______________(����)��
a.������� b.��עŨ���� c.�۲����������ٶ� d.������ѹ
��3����������������___________��ʵ��������������Ľ�ˮ��Ϊ__________(����a������b��)�ڡ�
��4��д��װ��C�з�����Ӧ�Ļ�ѧ����ʽ_______����װ�������¶ȼƿ����¶�Ϊ60~65 �棬ԭ����________��
��5����ȡ16.73 g POCl3��Ʒ�����Ƴ�100 mL��Һ��ȡ10.00 mL��Һ����ƿ�У�����3.2 mol��L-1��AgNO3��Һ10.00 mL��������ƿ�е���5��Fe2(SO4)3��Һ����0.20 mol��L-1��KSCN��Һ�ζ����ﵽ�ζ��յ�ʱ����KSCN��Һ10.00 mL(��֪��Ag++SCN-=AgSCN��)�������Fe2(SO4)3��Һ��������________����Ʒ��POCl3�Ĵ���Ϊ_____________��