��Ŀ����
���û�ѧ��Ӧԭ���о��������ȡ���ȵ��ʼ��仯����ķ�Ӧ����Ҫ����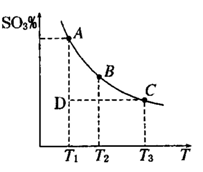
��1�����������У�SO2����������SO3�� 2SO2��g��+O2��g��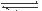 2SO3��g���������ϵ��SO3 �İٷֺ������¶ȵĹ�ϵ����ͼ��ʾ���������κ�һ�㶼��ʾƽ��״̬��������ͼʾ�ش��������⣬
2SO3��g���������ϵ��SO3 �İٷֺ������¶ȵĹ�ϵ����ͼ��ʾ���������κ�һ�㶼��ʾƽ��״̬��������ͼʾ�ش��������⣬
��2SO2��g��+O2��g��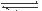 2SO3��g���ġ�H 0
2SO3��g���ġ�H 0
���>����<���������ں��¡���ѹ������������ƽ����ϵ��ͨ�뺤����ƽ�� �ƶ�����������ҡ�������
�����¶�ΪT1��T2����Ӧ��ƽ�ⳣ���ֱ�ΪK1��K2����K1 K2����Ӧ���е�״̬Dʱ�� 
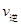 ���>����<����=����
���>����<����=����
��2�����ǵ����Ϻ����ḻ��һ��Ԫ�أ������仯�����ڹ�ũ ҵ������������������Ҫ���ã�
����ͼ��һ�����¶Ⱥ�ѹǿ��N2��H2��Ӧ����lmolNH3�����������仯ʾ��ͼ����д����ҵ�ϳɰ����Ȼ�ѧ����ʽ��
����H����ֵ�ú���ĸQ1��Q2�Ĵ���ʽ��ʾ��
�ڰ�������ˮ�õ���ˮ����25���£���a mol��L-1�İ�ˮ��b mol��L-1������������ϣ���Ӧ����Һ�����ԣ���c��NH4+�� c��Cl-�����>������<����=�������ú�a��b�Ĵ���ʽ��ʾ���û����Һ�а�ˮ�ĵ���ƽ�ⳣ�� .
��3����ˮ�к��д�����Ԫ�أ�����Ԫ�����ȣ���Ԫ����⣬���ں�ˮ�о��Ի���̬���ڣ���25���£���0��1L0.002mol��L-l��NaCl��Һ����μ���������0��1L0.002mol��L-l��������Һ���а�ɫ�������ɣ��ӳ����ܽ�ƽ��ĽǶȽ��Ͳ���������ԭ���� ����Ӧ�����Һ�м�������0��1L0.002mol��L-1��NaI��Һ�������������� �������������ԭ���ǣ������ӷ���ʽ��ʾ�� ��
����֪��25��ʱKSP��AgCl��=1.6��l0-10 KSP��AgI��=1.5��l0-16��
��1���٣� ���� (2��)
�ڣ� �� ��2�֣�
��2����N2(g)+3H2(g) 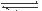 2NH3(g)��H=2(Q1-Q2)KJ/mol (3��)
2NH3(g)��H=2(Q1-Q2)KJ/mol (3��)
��= ��1�֣� 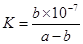 ��1�֣�
��1�֣�
��3��C(Ag+)��C(Cl-)��KSP(AgCl) ��1�֣� ��ɫ������ɻ�ɫ��1�֣�
AgCl(s)+I-(aq)=AgI(s)+Cl-(ag) ��2�֣�
���������������1��������ͼ��֪���¶�Խ�ߣ������ϵ��SO3�İٷֺ���ԽС��˵�������¶�ƽ�����淴Ӧ���У��������ƶ��������¶������ȷ�Ӧ�����ƶ������÷�Ӧ����ӦΪ���ȷ�Ӧ�����¡���ѹ������������ƽ����ϵ��ͨ�뺤�������Ӧ����Ӧ��������ֵ�Ũ�Ƚ��ͣ���ЧΪ����ѹǿ��ѹǿ����ƽ��������������ƶ����������ƶ���
���¶����ߣ�ƽ�������ȷ����ƶ��������淴Ӧ�ƶ���Kֵ��С��K1��K2��D״̬δ��ƽ�⣬�����ϵ��SO3�İٷֺ���С��ƽ��ʱ�ģ���Ӧ������Ӧ���У�����V����V����
��2������ͼ��֪��N2��H2��Ӧ����1molNH3�ų�������Ϊ��Q1-Q2��kJ���÷�Ӧ���Ȼ�ѧ��Ӧ����ʽΪN2(g)+3H2(g) 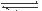 2NH3(g) ��H=2(Q1-Q2)KJ/mol ��
2NH3(g) ��H=2(Q1-Q2)KJ/mol ��
��amol��L��1��ˮ��Һ��bmol��L��1������Һ�������Ϻ�Ӧ�����Ȼ����Һ����Һ�д��ڵ���غ�,c��NH4������c��H����=c��Cl������c��OH����,��Һ������c��H����=c��OH����,����c��NH4����=c��Cl�D��,��ͺ���Һ��c(NH3��H2O )= 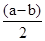 ,c��NH4����=c��Cl�D��=
,c��NH4����=c��Cl�D��=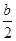 mol/L,c��H����=c��OH����=10-7mol/L,
mol/L,c��H����=c��OH����=10-7mol/L,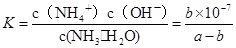 ,�𰸣�=��
,�𰸣�=��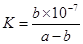
��3������Һ�����ӵ�Ũ����Qc=C(Ag+)��C(Cl-)��KSP(AgCl) ʱ���г�������������ѧʽ����ʾ���������Ӹ�������ͬ���ܶȻ�Խ���ܽ���Խ��AgCl��AgI���ܽ�ȴ��������ܽ�ȴ��ת��Ϊ�ܽ�ȸ�С�ģ�������AgClת��Ϊ�����ܵ�AgI������Ϊ��ɫ����ת��Ϊ��ɫ���������ӷ���ʽΪAgCl��s��+I���TAgI��s��+Cl����
���㣺��ѧƽ���Ӱ�����أ��Ȼ�ѧ����ʽ����ѧƽ�ⳣ���ĺ��壻��ѧƽ��״̬���жϣ����ܵ���ʵ��ܽ�ƽ�⼰����ת���ı���

���£��ݻ�Ϊ1 L���������£�����Է�������ת�����䷴Ӧ���̺�������ϵ��ͼ1��ʾ(��֪��2SO2(g)��O2(g) 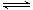 2SO3(g) ��H����196.6 kJ��mol��1)����ش��������⣺
2SO3(g) ��H����196.6 kJ��mol��1)����ش��������⣺
(1)д���ܱ�ʾ���ȼ���ȵ��Ȼ�ѧ����ʽ��______________________��
(2)��H2��__________kJ��mol��1��
��.��ҵ�ϳ����ô�����Ҵ��ϳ��л��ܼ�����������
CH3COOH(l)��C2H5OH(l)  CH3COOC2H5(l)��H2O(l) ��H����8.62 kJ��mol��1
CH3COOC2H5(l)��H2O(l) ��H����8.62 kJ��mol��1
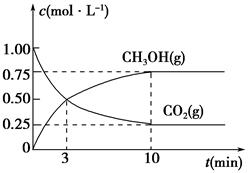
��֪CH3COOH��C2H5OH��CH3COOC2H5�ķе�����Ϊ118 �桢78 ���77 �档������������ͬʱ��ij�о�С������˶��ʵ�飬ʵ������ͼ��ʾ��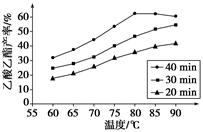
(1)���о�С���ʵ��Ŀ����___________________________________��
(2)60 ���·�Ӧ40 min��70 ���·�Ӧ20 min��ȣ�ǰ�ߵ�ƽ����Ӧ����________����(�С�ڡ��������ڡ����ڡ�)��
(3)��ͼ��ʾ����Ӧʱ��Ϊ40 min���¶ȳ���80 ��ʱ���������������½���ԭ�������_________________________________(д������)��
��.ú�����г����о���ͬ�¶���ƽ�ⳣ����Ͷ�ϱȼ���ֵ�����⡣
��֪��CO(g)��H2O(g) 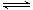 H2(g)��CO2(g)ƽ�ⳣ�����¶ȵı仯���±���
H2(g)��CO2(g)ƽ�ⳣ�����¶ȵı仯���±���
| �¶�/�� | 400 | 500 | 800 |
| ƽ�ⳣ��K | 9.94 | 9 | 1 |
�Իش��������⣺
(1)��800 �淢��������Ӧ���Ա��е����ʵ���Ͷ����ݷ�Ӧ��������������Ӧ�����ƶ�����________(ѡ�A��B��C��D��E��)��
| | n(CO) | n(H2O) | n(H2) | n(CO2) |
| A | 1 | 5 | 2 | 3 |
| B | 2 | 2 | 1 | 1 |
| C | 3 | 3 | 0 | 0 |
| D | 0.5 | 2 | 1 | 1 |
| E | 3 | 1 | 2 | 1 |
 2CO(g)ƽ�ⳣ��ΪK��
2CO(g)ƽ�ⳣ��ΪK����C(s)��H2O(g)
 CO(g)��H2(g) ƽ�ⳣ��ΪK1��
CO(g)��H2(g) ƽ�ⳣ��ΪK1����CO(g)��H2O(g)
 H2(g)��CO2(g) ƽ�ⳣ��ΪK2��
H2(g)��CO2(g) ƽ�ⳣ��ΪK2����K��K1��K2֮��Ĺ�ϵ��______________________________________��
�������������ʡ�����ȵ���Ҫԭ�ϣ�Χ�ƺϳɰ����ǽ�����һϵ�е��о�
��1�����������뵪������������������Ӧ�����Ƿ�Ӧ������ȴ����ͬ��
��֪��2H2 (g) + O2 (g) = 2H2O (g) ��H =" -483.6" kJ/mol
3H2 (g) + N2 (g)  2NH3 (g) ��H =" -92.4" kJ/mol
2NH3 (g) ��H =" -92.4" kJ/mol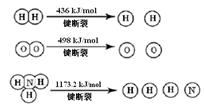
�������1 mol N��N����Ҫ���� kJ �� ���������л�ѧ�������������еĻ�ѧ���� ���ǿ�����������������������߷�Ӧ��������ͬ��
��2���̵��ǿ�ѧ�������о�����Ҫ���⡣��Ȼ���д�����Ȼ�Ĵ����̵����̣�N2 (g) + O2 (g) =" 2NO" (g) ��H =" +180.8" kJ/mol ����ҵ�ϳɰ������˹��̵���
�������̵ֹ���Ӧ��ƽ�ⳣ�������н�����ȷ���� ��
| ��Ӧ | �����̵� | ��ҵ�̵� | ||||
| �¶�/�� | 27 | 2000 | 25 | 350 | 400 | 450 |
| K | 3.84��10-31 | 0.1 | 5��108 | 1.847 | 0.507 | 0.152 |
A�������£������̵����������ܽ��У�����ҵ�̵��dz�������
B��������ģģ������̵����������
C����ҵ�̵��¶�Խ�ͣ�������������ӦԽ��ȫ
D��KԽ��˵���ϳɰ���Ӧ������Խ��
��3���ں��º����ܱ������а��ռס��ҡ������ַ�ʽ�ֱ�Ͷ��, ������Ӧ��3H2 (g) + N2 (g)
 2NH3 (g)��ü�������H2��ת����Ϊ40%��
2NH3 (g)��ü�������H2��ת����Ϊ40%��| | N2 | H2 | NH3 |
| �� | 1 | 3 | 0 |
| �� | 0.5 | 1.5 | 1 |
| �� | 0 | 0 | 4 |
��ƽ��ʱ���ס��ҡ�����������NH3�����������С˳��Ϊ ��
��4�������Ǻϳ������ԭ�ϣ�д��������������Ӧ����һ����������̬ˮ���Ȼ�ѧ����ʽ ��
���Ŵ�����Ⱦ���������أ��������ڡ�ʮ���塱�ڼ䣬����������(SO2)�ŷ�������8%����������(NOx)�ŷ�������10%��Ŀǰ������������Ⱦ�ж��ַ�����
��1�� ��CH4����ԭ��������������������������Ⱦ����֪��
��CH4(g)+4NO2(g) ="4NO(g)" + CO2(g) +2H2O(g) �SH=" -574" kJ��mol��1
��CH4(g) +4NO(g) =2N2(g) + CO2(g) + 2H2O(g) �SH=" -1160" kJ��mol��1
��H2O(g) = H2O(l) ��H=" -44.0" kJ��mol��1
д��CH4(g)��NO2(g)��Ӧ����N2 (g)��CO2 (g)��H2O(1)���Ȼ�ѧ����ʽ ��
��2������Fe2+��Fe3+�Ĵ����ã������¿ɽ�SO2ת��ΪSO42�����Ӷ�ʵ�ֶ�SO2����������֪��SO2�ķ���ͨ�뺬Fe2+��Fe3+����Һʱ������һ����Ӧ�����ӷ���ʽΪ4Fe2+ + O2+ 4H+ = 4Fe3+ + 2H2O������һ��Ӧ�����ӷ���ʽΪ ��
��3���û���̿��ԭ��������������йط�ӦΪ��C(s)+2NO(g) N2 (g)+CO2 (g) ��ij�о�С�����ܱյ���������У���������������䣬��������������Բ��ƣ�����NO�������Ļ���̿������(T1��)�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
N2 (g)+CO2 (g) ��ij�о�С�����ܱյ���������У���������������䣬��������������Բ��ƣ�����NO�������Ļ���̿������(T1��)�����·�Ӧ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
 Ũ��/mol��L��1 Ũ��/mol��L��1
| NO | N2 | CO2 | ||
| 0 | 1.00 | 0 | 0 | ||
| 10 | 0.58 | 0.21 | 0.21 | ||
| 20 | 0.40 | 0.30 | 0.30 | ||
| 30 | 0.40 | 0.30 | 0.30 | ||
| 40 | 0.32 | 0.34 | 0.17 | ||
| 50 | 0.32 | 0.34 | 0.17 |
�ڸ��ݱ������ݣ�����T1��ʱ�÷�Ӧ��ƽ�ⳣ��Ϊ ���� ��������λС������
��һ���¶��£�����NO����ʼŨ��������NO��ƽ��ת���� ������������䡱��С���� ��
�����и�������Ϊ�жϸ÷�Ӧ�ﵽƽ����� ������ ���������ĸ����
A��������ѹǿ���ֲ��䡡�� B�� 2v��(NO) = v��(N2)
C��������CO2������������� D�����������ܶȱ��ֲ���
��30minĩ�ı�ijһ��������һ��ʱ�䷴Ӧ���´ﵽƽ�⣬��ı������������ ��������ͼ�л���30min��40min�ı仯���ߡ�

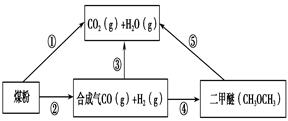
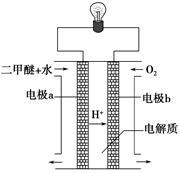
 CH3OH(g)��CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ����
CH3OH(g)��CO��ƽ��ת�������¶ȡ�ѹǿ�Ĺ�ϵ����ͼ��ʾ����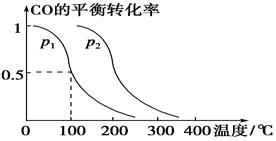
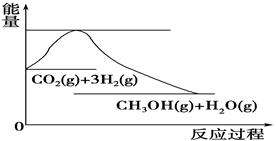
 ���ֱ���Ҫ��������436kJ��391KJ��946kJ����N2��H2��Ӧ����1mol NH3(g)���Ȼ�ѧ����ʽ��___________________.
���ֱ���Ҫ��������436kJ��391KJ��946kJ����N2��H2��Ӧ����1mol NH3(g)���Ȼ�ѧ����ʽ��___________________. 2CO2(g)+ N2(g)����H��0
2CO2(g)+ N2(g)����H��0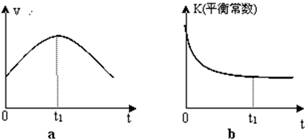
 N2O4(g) ��H����56.9 kJ/mol
N2O4(g) ��H����56.9 kJ/mol 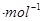 ��
�� ��һ283.0 kJ
��һ283.0 kJ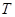 ��
�� ������300�棩��
������300�棩��
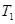 ʱ���ӷ�Ӧ��ʼ����Ӧ�ﵽƽ�⣬���ɼ״���ƽ������Ϊ��
ʱ���ӷ�Ӧ��ʼ����Ӧ�ﵽƽ�⣬���ɼ״���ƽ������Ϊ��
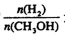 ����
����