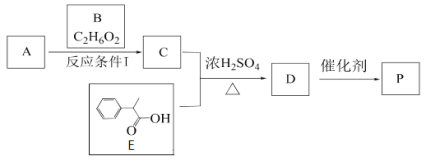
��Ŀ����
����Ŀ��ͼAװ�ó�����ʵ�����Ʊ�����
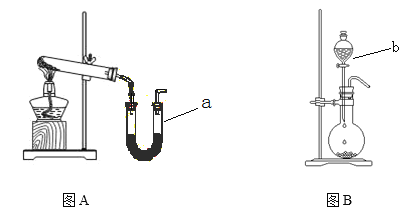
(1)д��ʵ�����ø�װ���Ʊ�O2��ѧ����ʽ __________________________________��
(2)�����ø�װ���Ʊ�����NH3���Թ��з���ҩƷ��_______________(�ѧʽ)������ a�з���ҩƷ������________ ��
(3)ͼBװ��ʵ���ҿ������Ʊ��������л�������_______������b������_________����ѧ������ͼBװ����Ũ��ˮ����ʯ���Ʊ�NH3����˵���÷�����ȡNH3��ԭ�� ��______________________________________________________________
(4)ѧ���װ�ͼ��ʾ̽����������
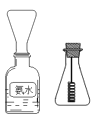
����һֻ��ƿ������Ũ��ˮ�Լ�ƿ���ռ����� ��Ȼ���ȵ�����״ͭ˿������ƿ�У�Ƭ�̣���ƿ�������Ϊ����ɫ������������ȷ����_________
A.��ͼ�ռ����������ð�ˮ���ܶȽ�С B.��ƿ�������
C.�ռ�����ʱ��Խ��������ɫ����Խ���� D.ͭ˿�ܱ��ֺ���
��ѧ���Ҷ�ѧ����ʵ����������飬��Ϊʵ���в����ĺ���ɫ��������ǿ����еĵ�����������ɵģ�����Ϊѧ���ҵ�˵���������������һ����ʵ��֤��ѧ���ҵ�˵���Ƿ���ȷ��_____________________________________��
���𰸡���2KMnO4![]() K2MnO4+MnO2+O2����2KClO3
K2MnO4+MnO2+O2����2KClO3![]() 2KCl+3O2���� NH4Cl��Ca(OH) 2 ��ʯ�� ��Ȳ��CH��CH ��Һ©�� ��ʯ���백ˮ�е�ˮ��Ӧ�����������ȴ�ʹ��ˮ�ֽ�����NH3 BD ����ȡһ����ƿ�������ȵ�����״ͭ˿������ƿ�й۲���
2KCl+3O2���� NH4Cl��Ca(OH) 2 ��ʯ�� ��Ȳ��CH��CH ��Һ©�� ��ʯ���백ˮ�е�ˮ��Ӧ�����������ȴ�ʹ��ˮ�ֽ�����NH3 BD ����ȡһ����ƿ�������ȵ�����״ͭ˿������ƿ�й۲���
��������
(1)��װ��Ϊ���������ȡ��������˿ɼ��ȸ�����ػ��������غͶ������̵Ļ���
(2)ʵ����װ��Ϊ��+��![]() ����������NH4Cl��Ca(OH)2������ȡ��������Ӧ����ˮ�����ü�ʯ�Ҹ��
����������NH4Cl��Ca(OH)2������ȡ��������Ӧ����ˮ�����ü�ʯ�Ҹ��
(3)ͼ2װ��Ϊ��+Һ�������������Ʊ��������л�������Ȳ������b��Һ©������ʯ���백ˮ�е�ˮ��Ӧ�����������ȴ�ʹ��ˮ�ֽ�����NH3��
(4)��A��Ũ��ˮ�ӷ��������ܶ�С�ڿ�����
B��������������ˮ����ƿ������
C��4NH3+5O2![]() 4NO+6H2O��2NO+O2=2NO2������
4NO+6H2O��2NO+O2=2NO2������
D���÷�ӦΪ���ȷ�Ӧ��ͭ˿�ܱ��ֺ��ȣ�
����ȡһ����ƿ�������ȵ�����״ͭ˿������ƿ�й۲����������������֤����������ɫ�����ɰ�����������ɣ������ǿ����е�����������ɡ�
(1)��װ��Ϊ���������ȡ�������ɼ��ȸ�����ػ��������غͶ������̵Ļ�����ѧ����ʽΪ2KMnO4![]() K2MnO4+MnO2+O2����2KClO3
K2MnO4+MnO2+O2����2KClO3![]() 2KCl+3O2�����ʴ�Ϊ��2KMnO4
2KCl+3O2�����ʴ�Ϊ��2KMnO4![]() K2MnO4+MnO2+O2����2KClO3
K2MnO4+MnO2+O2����2KClO3![]() 2KCl+3O2����
2KCl+3O2����
(2)ʵ����װ��Ϊ��+��![]() ����������NH4Cl��Ca(OH)2������ȡ��������ѧ����ʽΪCa(OH)2+2NH4Cl=CaCl2+2NH3��+2H2O�������е�ˮ���ü�ʯ�Ҹ���ʴ�Ϊ��NH4Cl��Ca(OH)2����ʯ�ң�
����������NH4Cl��Ca(OH)2������ȡ��������ѧ����ʽΪCa(OH)2+2NH4Cl=CaCl2+2NH3��+2H2O�������е�ˮ���ü�ʯ�Ҹ���ʴ�Ϊ��NH4Cl��Ca(OH)2����ʯ�ң�
(3)ͼ2װ��Ϊ��+Һ�������������Ʊ��������л�������Ȳ��CaC2+2H2O��Ca(OH)2+CH��CH��������b��Һ©������ʯ���백ˮ�е�ˮ��Ӧ�����������ȣ����Դ�ʹ��ˮ�ֽ�����NH3���ʴ�Ϊ����Ȳ��CH��CH����Һ©������ʯ���백ˮ�е�ˮ��Ӧ�����������ȴ�ʹ��ˮ�ֽ�����NH3��
(4)��A��Ũ��ˮ�ӷ��������ܶ�С�ڿ�������A����
B��������������ˮ����ƿ��������B��ȷ��
C�������ķ�ӦΪ4NH3+5O2![]() 4NO+6H2O��2NO+O2=2NO2���ռ�����ʱ��Խ������ƿ������Ũ��Խ�ͣ���������NO2��Ũ��Խ�ͣ���ɫԽdz����C����
4NO+6H2O��2NO+O2=2NO2���ռ�����ʱ��Խ������ƿ������Ũ��Խ�ͣ���������NO2��Ũ��Խ�ͣ���ɫԽdz����C����
D���÷�ӦΪ���ȷ�Ӧ��ͭ˿�ܱ��ֺ��ȣ���D��ȷ��
�ʴ�Ϊ��BD��
������ȡһ����ƿ�������ȵ�����״ͭ˿������ƿ�й۲��������������֤����������ɫ�����ɰ�����������ɣ������ǿ����е�����������ɣ�����ֺ���ɫ���壬˵���ҵ�˵���������ʴ�Ϊ������ȡһ����ƿ�������ȵ�����״ͭ˿������ƿ�й۲���

����Ŀ��ij��ѧС���������������������װ�ã���ͼ�����Ի������Ʊ�����ϩ��
��֪��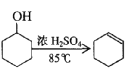 +H2O
+H2O
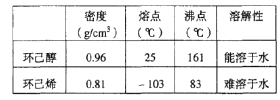
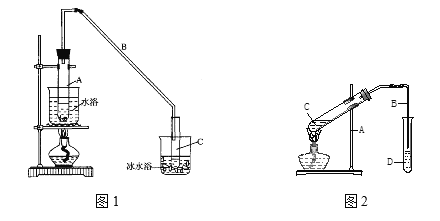
(1)��ͼ1��ʵ������ȡ����������װ�á�������˵������ȷ������____��
A���ұߵ��ܲ����뱥��̼������Һ
B���Թ�D����״�����²㣬����̼������Һ��ҪΪ���кͻӷ���������
C���Թ�C�м����Լ���˳���ǣ�2mLŨ������3mL�Ҵ���2mL������
D����Ӧ�������Թ�CҺ����ܻ���ɫ
(2)����ȡ���������Ĺ����У���Ũ������Ϊ������ȱ�㣬�����ظ�ʹ�ã����Ҹ���Ӧ�϶ࡣĿǰ�Ը÷�Ӧ�Ĵ����������µ�̽����������������������Һ��������˷�Ӧ�Ĵ����������ظ�ʹ�á�ʵ���������±���ʾ��������Ҵ��Ե����ʵ�����ϣ���
ͬһ��Ӧʱ�� | ͬһ��Ӧ�¶� | ||||
��Ӧ�¶�/�� | ת����(%) | ѡ����(%)* | ��Ӧʱ��/h | ת����(%) | ѡ����(%)* |
40 | 77.8 | 100 | 2 | 80.2 | 100 |
60 | 92.3 | 100 | 3 | 87.8 | 100 |
80 | 92.6 | 100 | 4 | 92.3 | 100 |
120 | 94.5 | 98.7 | 6 | 93.0 | 100 |
ѡ����100%��ʾ��Ӧ���ɵIJ���������������ˮ | |||||
���ݱ������ݣ�����________������ĸ��Ϊ�÷�Ӧ�����������
A.120��,4h B.80��,6h C. 60��,4h D.80��,4h
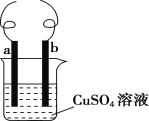
(3)�Ʊ�����ϩ��Ʒ��װ����ͼ2��
��12.5mL�����������Թ�A�У��ټ���1mLŨ���ᣬҡ�Ⱥ�������Ƭ��������������Ӧ��ȫ�����Թ�C�ڵõ�����ϩ��Ʒ��
��A�����Ƭ��������____________������B���˵�������е�������____________��
���Թ�C���ڱ�ˮԡ�е�Ŀ����_____________��
(4)�Ʊ�����ϩ��Ʒ
�ٻ���ϩ��Ʒ�к��л������������������ʵȡ����뱥��ʳ��ˮ�������á��ֲ㣬����ϩ��_________��(���ϻ���)����Һʱ��������Һ�����������������ԭ�����Һ©�����������⣬����________����Һ����_________ (������)ϴ�ӣ�
a��NaHSO4��Һ b��Na2CO3��Һ c��ϡH2SO4 d.��ˮ
�ڴ��Ʊ������У����뱥��ʳ��ˮ��������__________________��
�ڶԷ�������Ļ���ϩ�ٽ�������õ�����ϩ��Ʒ��Ϊ�����ֻ���ϩ��Ʒ�ʹ�Ʒ��ij��С����������¼��ַ�������������_________��
a�������Ը��������Һ b���ý���K c����Na2CO3��Һ d.��NaOH��Һ
����Ŀ��ȷ����8.2g�������������������ʵ��ռ���Ʒ�����500mL������Һ����0.1000molL1��������Һ�����к͵ζ��ⶨ���ռ���Ʒ�Ĵ��ȣ��Ը�������ش��������⣺
��1���ζ������У��۾�Ӧע��____________�����÷�̪��ָʾ���ﵽ�ζ��յ�ı�־��____________��
��2�����ݱ����ݣ������ռ���Ʒ�Ĵ�����_______________���ðٷ�����ʾ������С�������λ��
�ζ����� | ������Һ���(mL) | ������� | |
�ζ�ǰ�Ŀ̶�(mL) | �ζ���Ŀ̶�(mL)�� | ||
��һ�� | 10.00 | 0.40 | 20.50 |
�ڶ��� | 10.00 | 4.10 | 24.00 |
��3������ʵ�������Եζ��������ʲô�����������ƫ������ƫ����������Ӱ������
�ٹ۲���ʽ�ζ���Һ��ʱ����ʼ���ӣ��ζ��յ�ƽ�ӣ���ζ����________��
��������ƿ�ô���Һ��ϴ��Ȼ���ټ���10.00mL����Һ����ζ����________��