��Ŀ����
8��N��Cu��H��O��S��Mg�dz���������Ԫ�أ���1��Mgλ��Ԫ�����ڱ��������ڵ�IIA�壻N��O�Ļ�̬ԭ�Ӻ���δ�ɶԵ��Ӹ�����Ϊ
3��2��Cu�Ļ�̬ԭ�ӵ����Ų�ʽΪ1s22s22p63s23p63d104s1��
��2���á�����������գ�
| ���ԣ� Mg ��OH��2�� Cu��OH��2 | ��һ�����ܣ�O�� N | �۵㣺 MgS�� MgO | �ȶ��ԣ� H2S�� H2O |
��4����ҵ�ϲ�ȡ�����������ð�����ԭ����ͭ��ȡͭ��ͬʱ�õ���������Ⱦ�����壨����������д���÷�Ӧ�Ļ�ѧ����ʽ�����������ת�Ƶķ������Ŀ
 ��
��
���� ��1��Mg��12��Ԫ�أ�λ��Ԫ�����ڱ��������ڵ�IIA�壻Nԭ�ӵĺ�������Ų�Ϊ1s22s22p3��Oԭ�ӵĺ�������Ų�Ϊ1s22s22p4����������̬ԭ�Ӻ���δ�ɶԵ��Ӹ�����Ϊ3��2��Cu��29��Ԫ�أ�ͭ�Ļ�̬ԭ�ӵ����Ų�ʽΪ1s22s22p63s23p63d104s1��
��2��������Խǿ����������Ӧˮ����ļ���Խǿ��ͬ���ڵ�һ��������������A�͢�AԪ���쳣�����Ӿ��徧����Խ���۵�Խ�ߣ�����Ԫ�صķǽ�����Խǿ���⻯��Խ�ȶ���
��3���Ȼ�ѧ����ʽ��дҪע����Ӧ���������ľ۽�״̬�ͷ�Ӧ�ȣ�
��4��������ԭ����ͭ��ȡͭ��ͬʱ�õ���������Ⱦ�����壨��������ֻ���ǵ�����ˮ������Ȼ����ݵ���ת������������ת�Ƶ���Ŀ��
��� �⣺��1��Mg��12��Ԫ�أ�λ��Ԫ�����ڱ��������ڵ�IIA�壻N�ĺ�������Ų�Ϊ1s22s22p3��O�ĺ�������Ų�Ϊ1s22s22p4����������̬ԭ�Ӻ���δ�ɶԵ��Ӹ�����Ϊ3��2��Cu��29��Ԫ�أ�ͭ�Ļ�̬ԭ�ӵ����Ų�ʽΪ1s22s22p63s23p63d104s1��
�ʴ�Ϊ������IIA��3��2��1s22s22p63s23p63d104s1��
��2��������Խǿ����������Ӧˮ����ļ���Խǿ�������ԣ�Mg��Cu�����Լ��ԣ�Mg ��OH��2��Cu��OH��2����ͬ���ڵ�һ��������������A�͢�AԪ���쳣�����еĵ��ǵڢ�AԪ�أ����Ե����ܣ�O��N�������ӵİ뾶���������ӣ�����MgO�����ܴ���MgO���۵�ߣ����۵㣺MgS��MgO��
����Ԫ�صķǽ�����Խǿ���ǽ�����O��S�������⻯��Խ�ȶ�H2S��H2O���ʴ�Ϊ������������������
��3����ΪMg3N2��S������������ϡ����ɵõ��������Σ���25�桢101kPa�£���֪�÷�Ӧÿ����1mol H2SO4����akJ�������Ȼ�ѧ����ʽΪ��Mg3N2��S��+4 H2SO4��aq��=3MgSO4��aq��+��NH4�� 2SO4��aq����H=-4akJ/mol���ʴ�Ϊ��Mg3N2��S��+4 H2SO4��aq��=3MgSO4��aq��+��NH4�� 2SO4��aq����H=-4akJ/mol��
��4��������ԭ����ͭ��ȡͭ��ͬʱ�õ���������Ⱦ�����壨��������ֻ���ǵ�����ˮ���������Է�Ӧ����ʽΪ��2NH3+3CuO$\frac{\underline{\;\;��\;\;}}{\;}$3Cu+N2+3H2O���õ����ű�ʾ����ת��Ϊ�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
���� ���⿼��Ԫ�������ڱ��е�λ�úͺ�������Ų���Ԫ�������ɵ����֪ʶ���Ȼ�ѧ����ʽ����д���ۺ���ǿ���Ѷ��еȣ�

 ��ʦ����ָ���ο�ʱϵ�д�
��ʦ����ָ���ο�ʱϵ�д�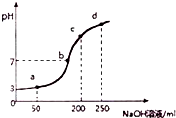 ����ʱ����100mL0.1mol?L-1NH4Al��SO4��2��Һ�еμ�0.2mol?L-1NaOH��Һ���õ���Һ��pH������NaOH��Һ����Ĺ�ϵ������ͼ��ʾ������˵������ȷ���ǣ�������
����ʱ����100mL0.1mol?L-1NH4Al��SO4��2��Һ�еμ�0.2mol?L-1NaOH��Һ���õ���Һ��pH������NaOH��Һ����Ĺ�ϵ������ͼ��ʾ������˵������ȷ���ǣ�������| A�� | a��b��c��d�ĸ��㣬ˮ�ĵ���̶�������a | |
| B�� | b����Һ�д���c��SO42-����c��Na+����c��NH4+�� | |
| C�� | ��0.1mol?L-1NH4Al��SO4��2��Һ�У�c��NH4+��+3c��Al3+��=0.199 mol?L-1 | |
| D�� | �μ�NaOH��Һ�ڼ䣬��Һ��NH4+��SO42-Ũ��֮��ʼ��Ϊ0.2mol?L-1 |
| A�� | ������Ư�۳���������ˮ�ľ����������������ߵ�����ԭ����һ���� | |
| B�� | Ϊ�˷�ֹ�����±��ĸ�֬ʳ�������������ʣ����ڰ�װ�������ʯ�� | |
| C�� | �������������п�飬�ɼ�������ĸ�ʴ���÷������������������������� | |
| D�� | �������pH��7����ˮ����Ҫ���ɴ����е�SO2��NO2����ɵ� |
| A�� | �����£�pH=9��NaHA��Һ��c��Na+����c��HA-����c��A2-��c��H2A�� | |
| B�� | Na2CO3��Һ��c��H+��-c��OH-��=c��HCO3-��+2c��CO32-��-c��Na+�� | |
| C�� | ��NaOH��Һ�е���HCOOH��Һ����Һ�Լ��ԣ�c��HCOO-����c��OH-����c��H+�� | |
| D�� | Ũ�Ⱦ�Ϊ0.1mol•L-1HF��Һ��0.1mol•L-1KF��Һ�������ϣ�c��F-��+c��HF��=0.2mol•L-1 |
| A�� | 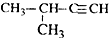 2-��-3-��Ȳ | B�� | 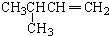 3-����ϩ | C�� | 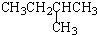 2-������ | D�� |  �������� |
| A�� | ��ij����ͨ����ˮ�У���ˮ��ɫ��ȥ��������һ������ϩ | |
| B�� | ��������ʱ�¶ȼ�ˮ�����ڷ�ӦҺ�� | |
| C�� | ʵ��������ϩʱ�¶ȼ�ˮ�����ڷ�ӦҺ�� | |
| D�� | �����������е���Ԫ��ʱ����������������NaOH��Һ��Ϲ��ȣ���ַ�Ӧ����ȴ�μ�AgNO3��Һ |
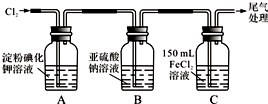
��1��ͨ��������A�е���������Һ����ɫ��Cװ���з�����Ӧ�����ӷ���ʽΪ��2Fe2++Cl2=2Fe3++2Cl-��
��2����ͨ������һ��ʱ���Bƿ����Һ����һ����SO32-������SO42-��������鷽��������Bƿ��Һ��Cl-��SO42-�Ĵ��ڣ�
| ʵ �� �� �� | Ԥ������ͽ��� |
| ����1��ȡ����Bƿ����Һ��һ�ɾ��Թ��У��μӹ���ϡ�����������BaCl2��Һ���� | ��������ɫ��������Bƿ��Һ�д���SO42-�� |
����2����ȡ����Bƿ����Һ���Թ�һ�ɾ��Թ��У��μӹ�����2mol/L HNO3��l mol/L Ba��NO3��2��Һ�������ã� | ������ɫ������ |
| ����3��ȡ����2���Թ��е��ϲ���Һ��һ�ɾ��Թ��У��μ�01mol/L AgNO3��Һ���� | ��������ɫ��������Bƿ��Һ�д���Cl-�� |
��3��Ϊȷ�ⶨͨ������һ��ʱ���Cƿ��ʣ��FeCl2�����ʵ�����ʵ�����£�
������250mL ��Һ����Cƿ��ȫ����Һȡ��ʢ��250mL����ƿ�У���ȷ���Ƴ�250mL��Һ��
ȷ��Cƿ�е���Һȫ��ȡ������������ʧ��������еIJ����ǽ�Cƿ�е���Һת�Ƶ�����ƿ������������ˮϴ��Cƿ2��3�Σ���ת������ƿ��
�ڵζ���ȷ��ȡ25.00mL������Һ����ƿ�У���0.20mol/L KMnO4��Һװ����ʽ�ζ��ܣ��ζ����յ㣬��¼���ݣ��ظ��ζ�2�Σ�ƽ������KMnO4��ҺV mL������Ӧ����ʽ��Fe2++MnO4-+H+-Fe3++Mn2++H2O��δ��ƽ��
�ۼ��㣺Cƿ��ʣ��FeCl2�����ʵ�����n��FeCl2��=0.01Vmol��
| A�� | 15g�������еĵ�������10NA�� | |
| B�� | 1mol����ϩ�к��е�̼��̼˫����Ϊ4NA�� | |
| C�� | ��״���£�1L���������еķ�����ΪNA/22.4 | |
| D�� | �����£�14g��ϩ�ͱ�ϩ�Ļ��������ԭ����Ϊ3NA�� |
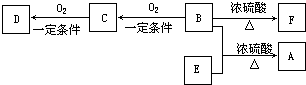 �л���A��C10H20O2������������ζ��������������ϴ���㲨�ķ������A��ͨ����ͼ��ʾ��ת����ϵ���Ƶã�
�л���A��C10H20O2������������ζ��������������ϴ���㲨�ķ������A��ͨ����ͼ��ʾ��ת����ϵ���Ƶã� ��
��