��Ŀ����
�����йط�Ӧ�ȵ�˵������ȷ���ǣ� ��
| A��һ����ѧ��Ӧ�Ƿ����ڳ����·�������÷�Ӧ�ġ�Hֵ�Ĵ�Сû�б�Ȼ��ϵ |
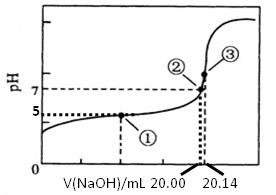
| B���к��ȡ�H����57.3kJ��mol��1������1.00L 1.00mol��L-1H2SO4��ϡ��NaOH��Һǡ����ȫ��Ӧ�ų�57.3kJ������ |
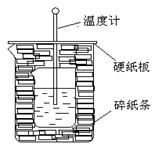
| C���õ������0.50mol��L��1���ᡢ0.55mol��L��1NaOH��Һ�����к��Ȳⶨ��ʵ�飬��ʹ��õ�ֵƫ�� |
| D����101kPaʱ��1molCH4��ȫȼ������CO2��ˮ�����ų���������CH4��ȼ���� |
A
�к�������һ�������£�ϡ��Һ�У�����Ӧ����1molˮʱ���ų�������������ѡ��B����ȷ����Ϊ�ڷ�Ӧʽ���ɵ���2molˮ��ѡ��C����ȷ����ʵ������û��Ӱ��ģ���һ�������£�1mol��ȼ����ȫȼ�������ȶ���������ʱ���ų���������ȼ���ȣ�ˮ���ȶ�״̬��Һ̬��ѡ��D����ȷ�������ȷ�Ĵ�ѡA��

��ϰ��ϵ�д�
�����Ŀ