��Ŀ����
����ý����������������Ṥҵ�Ĵ�����ijͬѧ������������ַ����о�����ý����ɡ�
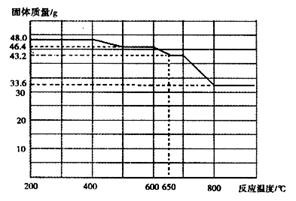
����һ��������װ�òⶨ����ý�ĺ�������ȷ������ɡ�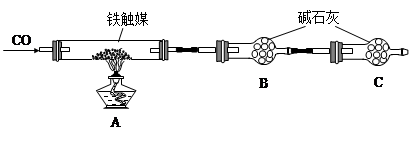
��������������ʵ�鷽���ⶨ����ý�ĺ�������ȷ������ɡ�
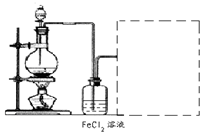
��1����������õ��IJ����������ձ�������������ͷ�ιܺ����� �� ��
��2����Ϊͨ��Cl2������������ҺB���л����� ��Ӱ��ⶨ�����
��3����Ϊͨ��Cl2�����Ҽ�����в���֣�����ҺB���п��ܺ���Cl2�������ʵ�鷽������Cl2���������ʵ�鱨�档
��ѡ�Լ���0.1mol��L��1����KMnO4��Һ����ɫʯ����Һ��Ʒ��ϡ��Һ������-KI��Һ��0.1moL��L��1KSCN��Һ
| ʵ����� | ʵ����������� |
| | |
��4���������C���������� ��
��5����ȡ15.2g����ý��������ʵ�顣��ַ�Ӧ��á������B������11.0g���������ý�Ļ�ѧʽ�ɱ�ʾΪ ��
��15�֣�
��1��250mL����ƿ ��2�֣�
��2��FeCl2����FeSO4����Fe2+ ��2�֣�
��3������һ��ʵ����� Ԥ������ͽ��� ȡ������ҺB���Թ��У��μ�
2-3����ɫʯ����Һ�����۲�������һ��ʱ����ڹ۲�����2�֣�����Һ�Ժ�ɫ��ɫ����ȥ������ҺB�к�Cl2��2�֣�������Һ����ɫ������ҺB������Cl2��2�֣�
��������ʵ����� Ԥ������ͽ��� ȡ������ҺB���Թ��У��μ�
2-3��Ʒ��ϡ��Һ�����۲�����2�֣�����Һ��ɫ������ҺB�к�Cl2��2�֣�������Һ����ɫ������ҺB������Cl2��2�֣�
��4����ֹ�����е�CO2��ˮ��������B�� ��2�֣�
��5��Fe4O5��2FeO��Fe2O3 ��3�֣�
���������������1�������Ϊ����һ��Ũ�ȵ���Һ����ʵ��ʱʹ�õ��Ⱥ�˳����Ҫ���������ձ�����������250mL����ƿ����ͷ�ιܣ���2����������ý����Ҫ�ɷ���������������������ﶼ�Ǽ������������������ϡ���ᣬ����ҺA����Ҫ�ɷ������������������������ᣬ��������ǿ�����ԣ��������Ӿ��л�ԭ�ԣ���ͨ��������Ŀ���ǽ�������������Ϊ�����ӣ����������㣬����ҺB�г���������������������Ȼ��������������ӵĴ��ڻ�Ӱ��ζ�ʱ���ı���Һ�������Ӱ��ⶨ�������3����ͨ����������������Һ��ֻ���������ӣ������������ӣ������ӶԼ�����Һ�п��ܺ��е������и��ţ���Ϊ���Ƕ����������ԣ�����ʹ����-KI��Һ��������˲���ѡ�����-KI��Һ���������Ĵ������Ҳ����ѡ�����Ը��������Һ��KSCN��Һ����Ϊ��������������ʱ�����Ա仯��ֻ�ܸ���������ˮ��Ӧ������������Ժʹ������ǿ���������ʵ�鷽��������һ��ȡ������ҺB���Թ��У��μ�2-3����ɫʯ����Һ�����۲�������һ��ʱ����ٹ۲���������Һ�Ժ�ɫ��ɫ����ȥ������ҺB�к�Cl2������Һ����ɫ������ҺB������Cl2����������ȡ������ҺB���Թ��У��μ�2-3��Ʒ��ϡ��Һ�����۲���������Һ��ɫ������ҺB�к�Cl2������Һ����ɫ������ҺB������Cl2����4�������B�м�ʯ�ҵ�Ŀ��������CO������ý��Ӧ���ɵĶ�����̼�������C�м�ʯ�ҵ�Ŀ���Ƿ�ֹ�����е�CO2��ˮ��������B�У���������ý��ɵIJⶨʵ�飻��5�����ڼ�ʯ���������CO����Ӧ��ֻ������CO2��������B���ӵ���������A�з�Ӧ���ɵ�CO2������������CO2����Է�������Ϊ44��m/M=n����n(CO2)=11.0g��44g/mol=0.25mol������̼Ԫ���غ�ɵù�ϵʽ��CO��CO2����μӷ�Ӧ��n(CO)= n(CO2)=0.25mol������n?M=m����m(CO)=0.25mol��28g/mol=7.0g������m(CO2)��m(CO)= 11.0g��7.0g=4.0g��������Ԫ�������غ�ɵã�����ý����Ԫ�ص�����Ϊ4.0g����������ý������Ϊ15.2g����������Ԫ�ص�����Ϊ15.2g��4.0g=11.2g������m/M=n����n(Fe)=11.2g��56g/mol=0.20mol����n(O)=4.0g��16g/mol=0.25mol������ý��������ԭ�ӵ����ʵ���֮��Ϊ0.20��0.25=4��5��������ý�Ļ�ѧʽΪFe4O5��2FeO��Fe2O3��
���㣺���黯ѧʵ�鷽������������ۣ��漰����һ�������Һ������������ҺB����Ҫ�ɷ֡���Ƽ�����ҺB���Ƿ��������ķ����������C�м�ʯ�ҵ����á�����ý��ѧʦ�IJⶨ�ͼ���ȡ�

 ȫ�ų��100��ϵ�д�
ȫ�ų��100��ϵ�д� Ӣ�ŵ��ϵ�д�
Ӣ�ŵ��ϵ�д�ijͬѧ������װ���Ʊ�������Cl2������: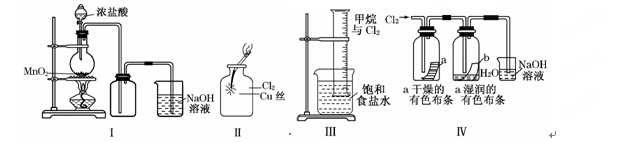
����˵����ȷ���ǣ� ��
| A����ͼ�У����MnO2������Ũ����Ϳ�ȫ�������� |
| B����ͼ�У�������ɫ���� |
| C����ͼ�У���Ͳ�з����˼ӳɷ�Ӧ |
| D���� ͼ��ʪ�����ɫ��������ɫ����Ũ������Һ�����ձ��У�����Һ�����ԣ������Cl2���� |
�������岻����ŨH2SO4�������
| A��CO2 | B��SO2 | C��NH3 | D��HC1 |
���б仯�У������ڻ�ѧ�仯���ǣ�������
| A��SO2ʹƷ����Һ��ɫ | B������ʹʪ��ĺ�ɫ������ɫ�� |
| C������̼ʹ��īˮ��ɫ | D����84������ҺʹijЩȾ����ɫ |
���л�ѧʵ����ʵ������۶���ȷ����
| ѡ�� | ʵ����ʵ | ���� |
| A | ��SO2ͨ�뺬HClO����Һ������H2SO4 | HClO�����Ա�H2SO4ǿ |
| B | �����ھƾ��ƻ����ϼ����ۻ��������� | ���������������۵������ |
| C | SiO2���Ժ�NaOH��Һ��HF��Һ��Ӧ | SiO2�������������� |
| D | ��SO2ͨ����ˮ�У����Ը��������Һ��ɫ | SO2����Ư���� |

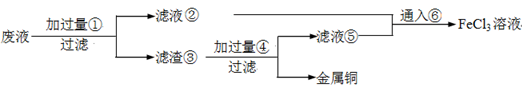
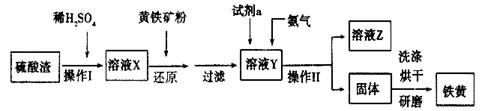
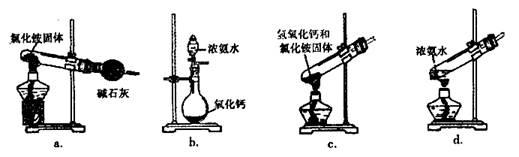
 �ķ����� ��
�ķ����� ��