��Ŀ����
ij�Ȼ�����Ʒ��������FeCl2���ʡ���Ҫ�ⶨ������Ԫ�ص�����������ʵ�鰴���²�����У�
������������̣��ش��������⣺
��1������I��������Һ�����õ��IJ����������ձ����������⣬�������� �� .�����������ƣ�
��2�����в�������ʹ������ҺŨ��ƫС����________________����д��ţ���
��δϴ���ձ��Ͳ�����
�ڶ���ʱ��������ƿ�Ŀ̶���
������Һǰ����ƿ������������ˮ
��ҡ�Ⱥ���Һ����ڿ̶��ߺ������ˮ����Һ����̶�������
��3����д��������ˮ���������ӷ���ʽ ��
��4����������Ƿ��Ѿ�ϴ�Ӹɾ��IJ����� ��
��5����ԭ��Ʒ����aΪ50g�����Ⱥ����ɫ��������bΪ3g������Ʒ����Ԫ�ص����������� ��
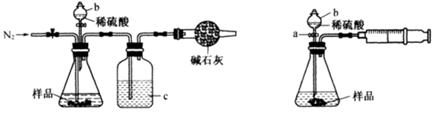
��1��250mL����ƿ��1�֣�����ͷ�ιܣ�1�֣�
��2���٢� ��2�֣�
��3��2Fe 2+��Cl2��2Fe 3+�� 2Cl�� ��2�֣�
��4��ȡ����ϴ��Һ���μ�AgNO3��Һ�����������ɣ���֤��ϴ�Ӹɾ� ��2�֣�
��5����Ԫ�ص�����������42%
���������������1����ͼ��֪������I�ǽ��������ᷴӦ����Һϡ�ͳ�250.00mL��Һ������Ҫ250mL����ƿ����ͷ�ιܣ�
��2���٢�ƫС����ƫ�ߣ�����Ӱ��
��3������ˮ������+2������Ϊ+3�ۣ�������ӦΪ2Fe 2++Cl2=2Fe 3++2Cl-��
��4��ϴ�ӵ�������������������Һ�����Ȼ����Һ�����Լ���Cl���������Ƿ�ϴ�Ӹɾ���ȡ���һ��ϴ��Һ���μӵμ�AgNO3��Һ�����������ɣ���֤��ϴ�Ӹɾ���
��5����250ml��Һ��ȡ25ml��Һ����õ�����ɫ��������bΪ3g����ԭ����250ml��Һ�õ�����ɫ��������Ϊ30g,��������������������112/116������30g��������������112/116��30g. ��Ʒ����Ԫ�ص�����������112/116��30g/50g��100�G=42�G
���㣺��Һ���ƺͳ�������

 ��ӡ�Ļ���ʱ����ϵ�д�
��ӡ�Ļ���ʱ����ϵ�д� ��ѧ�����ϵ�д�
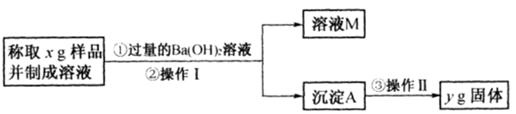
��ѧ�����ϵ�д���ʵ���Ҳⶨ̼������̼�����ƵĻ�����У�̼���Ƶ���������[�÷���w(Na2CO3)��ʾ]����ȡ�˻����5.lg������ˮ�У����250mL��Һ��
a��(10��)����һ����������w(Na2CO3)���û�ѧ��Ӧ��HCO3����CO32����ȫת��Ϊ��������ȡ�������������ɴ˼���������w (Na2CO3)��
��1����ȡ100 mL���ƺõ���Һ���ձ��У��μ�����������������Һ��HCO3����CO32����ȫת��Ϊ������Ӧѡ���Լ���___________ �����ţ���
| A��CaCl2 | B��MgSO4 | C����NaCI | D��Ba(OH)2 |
��3�����ˣ���ȡ����������˲�������Ҫ�IJ���������________________________��
��4��ϴ�ӳ���������ϴ�ӳ����IJ���_____________________________��
��5�������֣���ȡ����������Ϊ9.8g���ɴ˼���w(Na2CO3)������˲��У�����δ�����־ͳ���������w (Na2CO3)________________����ƫ���ƫС����Ӱ�죩��
b�����������ζ�����w(Na2CO3)��ȡ25.00 mL���ƺõ���Һ������ƿ�У��μ�2�η�̪�Լ���ҡ�ȣ���0.2000 mol/L��������еζ����յ㡣�ظ��˲���2�Σ�������������ƽ��ֵΪ20.00 mL�� [��֪���͵�̼����ҺPHΪ3.9]
��1����ȡ25.00 mL���ƺõ���Һ��Ӧѡ��_______________��������ɡ�
��2���жϵζ��յ��������_____________________���˹����з�Ӧ�����ӷ���ʽΪ__________________________________________________��
��3���˷����w(Na2CO3)=________%��������λС����
�ҹ�����ר�Һ�°����ĺ����Ƽ�Ļ�ѧԭ���ǽ�������̼ͨ�백ˮ���Ȼ��Ʊ�����Һ�У��仯ѧ��Ӧ����ʽΪ��NaCl+NH3+CO2+H2O=NaHCO3��+NH4Cl��
��1����ʵ��������������ԭ���ӷ�Ӧ������Һ�з����̼�����ƾ��壬Ӧѡ������װ���е� ��
��2��ʵ������̼�����ƾ����У����ܺ��е�����������Cl����NH4+��ʵ���Ҽ���Cl����ѡ�õ��Լ��ǡ���������һ���������ӵķ����� ������ţ���
| A����ˮ����ʪ��ĺ�ɫʯ����ֽ�����Թܿ� |
| B��������������Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿ� |
| C��������������Һ�����ȣ������̪�Լ� |
| D��������������Һ�����ȣ�������ɫʯ���Լ� |
����ý����������������Ṥҵ�Ĵ�����ijͬѧ������������ַ����о�����ý����ɡ�
����һ��������װ�òⶨ����ý�ĺ�������ȷ������ɡ�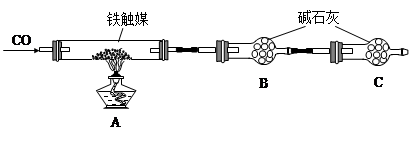
��������������ʵ�鷽���ⶨ����ý�ĺ�������ȷ������ɡ�
��1����������õ��IJ����������ձ�������������ͷ�ιܺ����� �� ��
��2����Ϊͨ��Cl2������������ҺB���л����� ��Ӱ��ⶨ�����
��3����Ϊͨ��Cl2�����Ҽ�����в���֣�����ҺB���п��ܺ���Cl2�������ʵ�鷽������Cl2���������ʵ�鱨�档
��ѡ�Լ���0.1mol��L��1����KMnO4��Һ����ɫʯ����Һ��Ʒ��ϡ��Һ������-KI��Һ��0.1moL��L��1KSCN��Һ
| ʵ����� | ʵ����������� |
| | |
��4���������C���������� ��
��5����ȡ15.2g����ý��������ʵ�顣��ַ�Ӧ��á������B������11.0g���������ý�Ļ�ѧʽ�ɱ�ʾΪ ��