��Ŀ����
����Ŀ�������������ʵ�̽��Ҫ�õ����ѧ֪ʶ��
(1)�±��г������������е�Ħ���������ڱ�����д����Ħ�����������������
���� | ���������� | �ѽ�ʿ���� | �л����� |
Ħ���� | ����þ(MgO) | ̼���(CaCO3) | �������� (SiO2) |
Ħ�������������(ָ�ᡢ��Ρ��������������������) | _________ | _________ | _________ |
(2)�����Ʋ�,����Ħ�������ܽ�����_________(��������������������)��
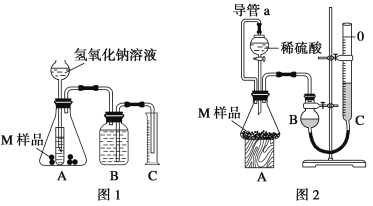
(3)�����е�Ħ����̼��ƿ�����ʯ��ʯ���Ʊ���ijѧ�������һ��ʵ�����Ʊ�̼��Ƶ�ʵ�鷽����������ͼ���£�
ʯ��ʯ![]() ��ʯ��
��ʯ��![]() ʯ��ˮ
ʯ��ˮ![]() ̼���
̼���
��д�������������йط�Ӧ�Ļ�ѧ����ʽ��
��___________________ ��___________________��
���𰸡����������� �� ���������� ���� CaCO3![]() CaO+CO2�� CaO+H2O=Ca(OH)2
CaO+CO2�� CaO+H2O=Ca(OH)2
��������
(1)�������ʵ�Ԫ����ɺ���������ص㣬�ж����ʵ����
(2)Ħ������Ϊ��Ħ���õģ���������ˮ��
(3)���ݷ�Ӧ��������������д��ѧ����ʽ��
(1)����þ����þ��������Ԫ����ɵĻ�������������MgO���ᷴӦ�����κ�ˮ�����ڼ��������
̼������ɽ������Ӻ����������ɵĻ���������Σ�
����������ֻ�й�Ԫ�غ���Ԫ����ɵĻ�����������������Ӧ�����κ�ˮ���������������
(2)����Ħ������Ϊ��Ħ���õģ�Ӧ����������ˮ�����ʣ�
(3)ʯ��ʯ����Ҫ�ɷ�̼��ƣ����·ֽ����������ƺͶ�����̼����ʯ�Һ�ˮ��Ӧ�����������ƣ��������ƺ�̼���Ʒ�Ӧ����̼��Ƴ������������ơ�
�ٷ�Ӧ�ķ���ʽΪCaCO3![]() CaO+CO2����
CaO+CO2����
�ڷ�Ӧ�ķ���ʽΪCaO+H2O=Ca(OH)2��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��T Kʱ����2.0 L�����ܱ������г���0.10 mol COCl2��������ӦCOCl2(g)![]() Cl2(g)��CO(g)������һ��ʱ���Ӧ�ﵽƽ�⡣��Ӧ�����в�õIJ������ݼ��±���
Cl2(g)��CO(g)������һ��ʱ���Ӧ�ﵽƽ�⡣��Ӧ�����в�õIJ������ݼ��±���
t/s | 0 | 2 | 4 | 6 | 8 |
n(Cl2)/mol | 0 | 0.030 | 0.039 | 0.040 | 0.040 |
����˵����ȷ����( )
A. ���������������䣬�����¶ȣ�ƽ��ʱc(Cl2)��0.038 mol��L��1����Ӧ����H��0
B. ��Ӧ��ǰ2 s��ƽ������v(CO)��0.015 mol��L��1��s��1
C. ���������������䣬��ʼʱ�������г���0.12 mol COCl2��0.06 mol Cl2��0.06 mol CO����Ӧ�ﵽƽ��ǰ�����ʣ�v����v��
D. ���������������䣬��ʼʱ�������г���0.10 mol Cl2��0.08 mol CO���ﵽƽ��ʱ��Cl2��ת���ʴ���60%