��Ŀ����
����Ŀ������������ظ�����(PMPS)�Ļ�ѧ���Ϊ![]() ������������ˮ����������ֳҵ�еĹ������������������������ȡ�ijУ���ʵ���Ʊ�PMPS��̽������ʡ��մ��������⣺
������������ˮ����������ֳҵ�еĹ������������������������ȡ�ijУ���ʵ���Ʊ�PMPS��̽������ʡ��մ��������⣺
��1������ͬѧ�Ʊ�PMPS��Һ�IJ������£�
����1.��30%![]() ��Һ�ñ�ˮ��ȴ�������л����μ�Ũ���ᣬ�õ�
��Һ�ñ�ˮ��ȴ�������л����μ�Ũ���ᣬ�õ�![]() ��
��![]() �Ļ����Һ��
�Ļ����Һ��
����2.����![]() ���ȶ�������������
���ȶ�������������![]() ������ҺpHΪ2~3ʱ�õ���ɫ��PMPS��Һ��
������ҺpHΪ2~3ʱ�õ���ɫ��PMPS��Һ��
��������1��![]() ��Һ�������ñ�ˮ��ȴ��Ŀ����____________;����Ũ���ᷢ����Ӧ(���淴Ӧ���Ļ�ѧ����ʽΪ____________��
��Һ�������ñ�ˮ��ȴ��Ŀ����____________;����Ũ���ᷢ����Ӧ(���淴Ӧ���Ļ�ѧ����ʽΪ____________��
��ʵ��������pH��ֽ�ⶨ��ҺpH�ķ�����____________��
��2������ͬѧȡ����PMPS��Һ�����Թ��У������е���2��![]() ��Һ����Һ��Ϊ��ɫ��˵�������ԣ�
��Һ����Һ��Ϊ��ɫ��˵�������ԣ�![]() ____________
____________![]() (����>������<������
(����>������<������
��3��PMPS�����ڿ�ʴͭ������ͬѧ��PMPS��Һ�м������������Ტ����ͭ������������Ҫ��Ӧ�Ļ�ѧ����ʽΪ________________��
��4�����ױ�����������NaClO��PMPS����Ч�ѳ������е�NO,ijͬѧ�������װ�ý�����֤��
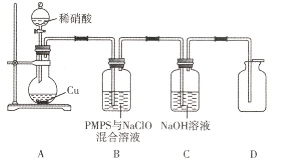
��װ��A�з�����Ӧ�����ӷ���ʽΪ________________
��ʢװϡ���������������____________;װ��C��������____________
����˵��D(������ɫ���м�������NO��������____________
���𰸡���ֹ���ʱ�¶����ߵ���H2O2�ֽ� H2O2+H2SO4![]() H2SO5+H2O ��pH��ֽ���ڸ���ྻ�IJ���Ƭ�ϣ��ò�����պȡ��Һ����pH��ֽ��,��ɫ�������ɫ���Ƚ� >
H2SO5+H2O ��pH��ֽ���ڸ���ྻ�IJ���Ƭ�ϣ��ò�����պȡ��Һ����pH��ֽ��,��ɫ�������ɫ���Ƚ� > ![]() ��
��![]()
![]() ��Һ©�� �������������ɵ�NO2(��NO2������NO) ƿ�����弸����ɫ
��Һ©�� �������������ɵ�NO2(��NO2������NO) ƿ�����弸����ɫ
��������
(2)������ԭ��Ӧ����������������>�������
(4)װ��A��ϡ������ͭ��Ӧ����NO������װ��B��PMPS��NaClO��Ӧ��NO��������������NO3-��NO2-��NO2�ȣ�װ��C��NaOH�������շ�Ӧ���ɵ�NO2��Dװ�ÿɼ���NO�Ƿ���ȫ��Ӧ��
(1)��Ũ��������Һ��Ϸ��ȣ�����H2O2�����ֽ⣬���轫ŨH2O2��Һ���ñ�ˮ��ȴ��������H2SO5��H2SO4�����Һ����֪H2O2��H2SO4��Ӧ����H2SO5��H2O������ʽΪH2O2+H2SO4![]() H2SO5+H2O��
H2SO5+H2O��
�ڲⶨ��ҺpH��ע���ø����pH��ֽ���ýྻ�IJ�����պȡ��Һ����pH��ֽ����ɫ�������ɫ���Ƚ϶�����
(2)��Һ��Ϊ��ɫ��˵������Mn2+��MnO4-��ת���������ԣ�������������������������ԣ�HSO5- > MnO4-��
(3)KHSO5����ǿ�����ԣ�ʴ��ͭ����H2O2��Cu��Ӧ����д����Ӧ�ķ���ʽΪ��![]() ��
��![]()
(4)��ͭ��ϡ���ᷴӦ��������ͭ��NO�����ӷ���ʽΪ![]() ��
��
�ڸ��������Ľṹ��֪��Ϊ��Һ©����KHSO5��NaClO������ǿ�����ԣ��ɽ�NO����ΪNO3-��NO2-��NO2����NaOH��Һ�����շ�Ӧ���ɵ�NO2�Լ�����δ��Ӧ��NO��
�������β�����н϶�NO����ƿ�ڻ�������������������ɺ���ɫ��NO2��

����Ŀ�������������ʵ�̽��Ҫ�õ����ѧ֪ʶ��
(1)�±��г������������е�Ħ���������ڱ�����д����Ħ�����������������
���� | ���������� | �ѽ�ʿ���� | �л����� |
Ħ���� | ����þ(MgO) | ̼���(CaCO3) | �������� (SiO2) |
Ħ�������������(ָ�ᡢ��Ρ��������������������) | _________ | _________ | _________ |
(2)�����Ʋ�,����Ħ�������ܽ�����_________(��������������������)��
(3)�����е�Ħ����̼��ƿ�����ʯ��ʯ���Ʊ���ijѧ�������һ��ʵ�����Ʊ�̼��Ƶ�ʵ�鷽����������ͼ���£�
ʯ��ʯ![]() ��ʯ��
��ʯ��![]() ʯ��ˮ
ʯ��ˮ![]() ̼���
̼���
��д�������������йط�Ӧ�Ļ�ѧ����ʽ��
��___________________ ��___________________��
����Ŀ����ͼ�����ʼ䷢����ѧ��Ӧ����ɫ�仯���±�ѡ���е����ʶ�Ӧ��ȷ����(����)
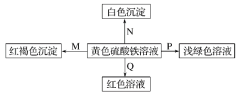
ѡ�� | M | N | P | Q |
A | NH3��H2O | Ba(OH)2 | ͭ | ʯ����Һ |
B | Na | BaCl2 | FeO | KSCN |
C | NaOH | Ba(NO3)2 | � | KSCN |
D | Na2O2 | MgCl2 | Fe | ʯ����Һ |
A.AB.BC.CD.D