��Ŀ����
����Ŀ�����й�ϵʽ�������( �� )
A. CO2��ˮ��Һ��c(H+)��c(HCO3-)��2c(CO32-)
B. ��Ũ�ȵ�HCN��Һ��NaOH��Һ�������ϣ�������ҺpH>7������Һ������Ũ�ȣ�c(Na+)��c(CN-) ��c(OH-)��c(H+)
C. NaHCO3��Һ�д���ˮ��ƽ����HCO3-+H2OH2CO3+OH-
D. ��������HX��HY��Ϻ���Һ�е�c(H+)Ϊ(KaΪ����ƽ�ⳣ��) ![]() +
+![]() + c(OH-)
+ c(OH-)
���𰸡�D
��������
A��CO2��ˮ��Һ�У�̼�Ჿ�ֵ���������ӣ���Һ�����ԣ����������ӻ�����ˮ�ĵ��롢HCO3-�ĵ��룬��c(H+)��c(HCO3-)�����ڵڶ������뼫������c(HCO3-)����c(CO32-)�����Ը���Һ������Ũ�ȴ�СΪ��c(H+)��c(HCO3-)��2c(CO32-)����A��ȷ��B����Ũ�ȵ�HCN��Һ��NaOH��Һ�������ϣ�������ҺpH��7����c(OH-)��c(H+)�����ݵ���غ�ɵã�c(Na+)��c(CN-)������Һ������Ũ�ȴ�СΪ��c(Na+)��c(CN-)��c(OH-)��c(H+)����B��ȷ��C��HCO3-��ˮ������H2CO3��OH-��ˮ�ⷽ��ʽΪHCO3-+H2OH2CO3+OH-����C��ȷ��D����������HX��HY��Ϻ��ݶ��ߵĵ���ƽ�ⳣ����֪��Һ�е�c(H+)Ϊ(KaΪ����ƽ�ⳣ��)��c(H+)=![]() =
=![]() ����D����ѡD��
����D����ѡD��

����Ŀ����1����Ӧ3Fe��s��+4H2O��g��![]() Fe3O4��s��+4H2��g����һ�ݻ��ɱ���ܱ������н��У��Իش�
Fe3O4��s��+4H2��g����һ�ݻ��ɱ���ܱ������н��У��Իش�
������Fe�������䷴Ӧ����____����������������������������С������ͬ����
���������������Сһ�룬�䷴Ӧ����____��
������������䣬����He���䷴Ӧ����____��
������ѹǿ���䣬����He���䷴Ӧ����_____��
��2����������Ϊ���������ں��º����ܱ������г���һ������NO��NH3����һ�������·�����Ӧ��6NO��g��+4NH3��g��![]() 5N2��g��+6H2O��g����
5N2��g��+6H2O��g����
����˵���÷�Ӧ�Ѵﵽƽ��״̬�ı�־��____������ĸ��ţ�
a.��Ӧ����5v��NH3��=4v��N2��
b.��λʱ����ÿ����5mol N2��ͬʱ����4mol NH3
c.������N2�����ʵ�������������ʱ��������仯
d.������n(NO����n��NH3����n��N2����n��H2O��=6��4��5��6
��ij��ʵ���в��������NO��N2�����ʵ�����ʱ��仯��ͼ��ʾ��ͼ��v��������v���棩��ȵĵ�Ϊ_____��ѡ����ĸ����

��3��298Kʱ������֪���ɱ�״����2.24LNH3ʱ�ų�����Ϊ4.62kJ��д���ϳɰ���Ӧ���Ȼ�ѧ����ʽ____��
��4��һ�������£���2L�ܱ������ڣ���Ӧ2NO2��g��=N2O4��g����H=-180kJ��mol-1��n��NO2����ʱ��仯���±���
ʱ��/s | 0 | 1 | 2 | 3 | 4 | 5 |
n(NO2)/mol | 0.040 | 0.020 | 0.010 | 0.005 | 0.005 | 0.005 |
��NO2��ʾ0��2s�ڸ÷�Ӧ��ƽ���ٶ�____���ڵ�5sʱ��NO2��ת����Ϊ____�������ϱ����Կ��������ŷ�Ӧ���У���Ӧ������С����ԭ����____��
����Ŀ���������NO2����һ����Ҫ�Ĵ�����Ⱦ�������д�����
��1����������������ʱ������N2��O2��Ӧ����NO���䷴Ӧ�����е������仯���£�
��Ӧ | N2(g)��2N(g) | O2(g)��2O(g) | N(g)��O(g)��NO(g) |
��Ӧ�� | ��H1 | ��H2 | ��H3 |
����ֵkJ/mol | 945 | 498 | 630 |
�١�H1___0����H3____0��������>������<����
��N2��g��+O2��g��=2NO��g����H=____kJ��mol-1��
��2�����ü������ԭ���������֪��
CH4��g��+4NO2��g��==4NO��g��+CO2��g��+2H2O��g����H=-574kJ��mol-l
CH4��g��+4NO��g��==2N2��g��+CO2��g��+2H2O��g��AH=-1160 kJ��mol-l
H2O��l��=H2O��g����H=+44kJ��mol-l
CH4��NO2��Ӧ����N2��H2O(l)���Ȼ�ѧ����ʽΪ_______��
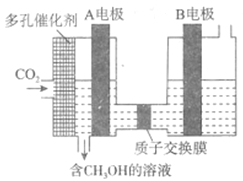
��3��DZͧ��ʹ�õ�Һ��-Һ��ȼ�ϵ�ع���ԭ����ͼ��ʾ��

���缫a������______��
���������Һ��OH-������_____�ƶ��������缫a�������缫b������
���缫b�ĵ缫��ӦʽΪ_____��
��4����ͨ��NH3��NaClO��Ӧ���Ƶû��ȼ���£�N2H4�����÷�Ӧ�Ļ�ѧ��Ӧ����ʽ��_____��