��Ŀ����
����Ŀ��I.��1����ϵͳ����������![]() ___��
___��
��2����Է�������Ϊ114����һ�ȴ���ֻ��һ�ֵ������Ľṹ��ʽ___��
��3��ij��1���Ӻ���50�����ӣ�����ֻ����һ�ֽṹ��Ȳ������õ���������ļ���ʽΪ__��
��.��1�����л���B�����������г��ȼ�գ�ʵ��������7.2gH2O��13.2gCO2����������10.08L(��״��)����������и�Ԫ�ص�ԭ�Ӹ���֮����___��
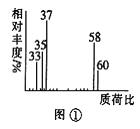
��2���������Dzⶨ���л����������Է����������õ���ͼ����ʾ����ͼ��������Է�������Ϊ___�������ʵķ���ʽ��___��
��3�����ݼۼ����ۣ�Ԥ��A���ܵĽṹ��ʽ��___��д������2�֣���
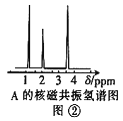
��4���˴Ź��������ܶ��л�������в�ͬλ�õ���ԭ�Ӹ�����ͬ�ķ�ֵ���źţ������ݷ�ֵ����ȷ����������ԭ�ӵ��������Ŀ�����ⶨ���л���A�ĺ˴Ź���������ͼ�ڣ���A�Ľṹ��ʽΪ��____��
���𰸡�2��4-����-3-�һ�-3-�� CH3��3CC��CH3��3 ![]() ��
��![]() N(C)��N(H)��N(O)=3��8��1 60 C3H8O CH3CH2CH2OH��CH3CH2OCH3��
N(C)��N(H)��N(O)=3��8��1 60 C3H8O CH3CH2CH2OH��CH3CH2OCH3��![]() CH3CH2OCH3
CH3CH2OCH3
��������
I����1�����л���Ϊϩ����ѡȡ����̼̼˫�����̼��Ϊ���������л�������Ϊ��ϩ��Ȼ�����ϩ��������ԭ��д�������ƣ���2������������ͨʽ����Է������������ۣ�Ȼ��ȷ���������ķ���ʽ���ٸ���һ�ȴ���ֻ��һ��ȷ�����л���Ľṹ��ʽ����3������ֻ����һ�ֽṹ��Ȳ������õ���˵�����л���Ϊϩ����������������ϩ����������ͨʽ�������к��еĵ��������۵ó�����ʽ���ٸ������Ӧ��Ȳ��ֻ��1�ֽṹд����ṹ��ʽ������ʽ��
��1������Ԫ���غ���ж��л���������һ������C��H����Ԫ�أ���������13.2g�Ķ�����̼��CԪ��������Ϊ������������CԪ������������7.2g��ˮ��HԪ��������Ϊ������������HԪ������������Ԫ�ص�ȷ��Ҫ���ݶ�����̼��ˮ�е���Ԫ������֮������������Ԫ�ص���������������ǰ�ߴ����л�����������Ԫ�أ�������ȣ����л�������û������Ԫ�أ�Ȼ��������Ե����ʵ��������ݸ�Ԫ�ص����ʵ���֮�ȿ��Ʋ�ʵ��ʽ�����ʽ������2�������л���ԭ�Ӹ�����ֵ��ȷ�����ʽ�������Է���������ȷ���л������ʽ����3�������л������ʽ��ϼۼ����ۿ�ȷ���л���Ŀ��ܽṹ����4���л���A�����������ֲ�ͬ��ѧ��������ԭ�ӣ�ӦΪ�����ѣ��������������⡣
I����1��![]() �У�����̼̼˫�����̼������6��C������Ϊ��ϩ��ѡȡ����֧������̼��Ϊ�������Ӿ���̼̼˫�������֧�������С�Ҷ˿�ʼ��ţ�̼̼˫����3��C����2��4��C������1��������3��C����1���һ������л�������Ϊ��2��4-����-3-�һ�-3-��ϩ���ʴ�Ϊ��2��4-����-3-�һ�-3-��ϩ��
�У�����̼̼˫�����̼������6��C������Ϊ��ϩ��ѡȡ����֧������̼��Ϊ�������Ӿ���̼̼˫�������֧�������С�Ҷ˿�ʼ��ţ�̼̼˫����3��C����2��4��C������1��������3��C����1���һ������л�������Ϊ��2��4-����-3-�һ�-3-��ϩ���ʴ�Ϊ��2��4-����-3-�һ�-3-��ϩ��
��2����Է�������Ϊ114��������������������ϩ����Ȳ���ȣ�����![]() =8��2��֪�����л���Ϊ�����������ʽΪC8H18��һ�ȴ���ֻ��һ�ֵ�������˵�����л�������е���ԭ��λ��ֻ��1�֣�����������Ľṹ��ʽΪ����CH3��3CC��CH3��3���ʴ�Ϊ����CH3��3CC��CH3��3��
=8��2��֪�����л���Ϊ�����������ʽΪC8H18��һ�ȴ���ֻ��һ�ֵ�������˵�����л�������е���ԭ��λ��ֻ��1�֣�����������Ľṹ��ʽΪ����CH3��3CC��CH3��3���ʴ�Ϊ����CH3��3CC��CH3��3��
��3��ij��1���Ӻ���50�����ӣ�����ֻ����һ�ֽṹ��Ȳ������õ������л������Ϊ������ϩ����ͨʽΪ��CnH2n����CnH2n+2�������к��еĵ���Ϊ����6n+2n=50���6n+2n+2=50�����ݢٿɵã�n=![]() �������������ݢڿɵã�n=6�����Ը��л���Ϊ����������ʽΪ��C6H14��������ֻ����һ�ֽṹ��Ȳ�������ã��������������̼��������̼̼������ֻ��һ�����������������������Ľṹ��ʽΪ��CH3CH2C��CH3��3��CH3CH2CH��CH3��CH2CH3����д�ɼ���ʽΪ��
�������������ݢڿɵã�n=6�����Ը��л���Ϊ����������ʽΪ��C6H14��������ֻ����һ�ֽṹ��Ȳ�������ã��������������̼��������̼̼������ֻ��һ�����������������������Ľṹ��ʽΪ��CH3CH2C��CH3��3��CH3CH2CH��CH3��CH2CH3����д�ɼ���ʽΪ��![]() ��
��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��![]()
��1��n��C��=n��CO2��=![]() =0.3mol��n��H��=2n��H2O��=
=0.3mol��n��H��=2n��H2O��=![]()
![]() =0.45mol��������ԭ���غ��֪�л����к��У�n��O��=0.4mol+0.3mol��2-0.45mol��2=0.1mol�����л�����N��C����N��H����N��O��=0.3mol��0.8mol��0.1mol=3��8��1���ʴ�Ϊ��N(C)��N(H)��N(O)=3��8��1��
=0.45mol��������ԭ���غ��֪�л����к��У�n��O��=0.4mol+0.3mol��2-0.45mol��2=0.1mol�����л�����N��C����N��H����N��O��=0.3mol��0.8mol��0.1mol=3��8��1���ʴ�Ϊ��N(C)��N(H)��N(O)=3��8��1��
��2����n��C����n��H����n��O��=3��8��1�������л��������ʵ��ʽΪC3H8O�����ʺɱȿ�֪��Է�������Ϊ60�������ʽΪC3H8O���ʴ�Ϊ��60��C3H8O��
��3���л���ķ���ʽΪC3H8O�������п��ܴ���C-C��C-H��C-O��O-H�Ȼ�ѧ�������ܵĽṹ��ʽ��CH3CH2CH2OH��CH3CH2OCH3��CH3CHOHCH3���ʴ�Ϊ��CH3CH2CH2OH��CH3CH2OCH3��![]() ��
��
��4���л���A�����������ֲ�ͬ��ѧ��������ԭ�ӣ�ӦΪ�����ѣ��������������⣬����A�Ľṹ��ʽΪCH3CH2OCH3���ʴ�Ϊ��CH3CH2OCH3��

����Ŀ�������л���Ӧ���͵������жϲ���ȷ���ǣ� ��
ѡ�� | �л���Ӧ | ��Ӧ���� |
A | ����H2��Ӧ���ɻ����� | �ӳɷ�Ӧ |
B | ������������Ϲ��ձ�ը | ȡ����Ӧ |
C | ��ϩ�����CCl4��Һ��Ӧ | ȡ����Ӧ |
D | ��ϩʹ���Ը��������Һ��ɫ | ������Ӧ |
A.AB.BC.CD.D