��Ŀ����
����Ŀ����9.0 gͭ�����Ļ����Ͷ��100 mLϡ�����в����ȣ���ַ�Ӧ��õ���״����1.12 L NO��ʣ��4.8 g��������������100 mL��Ũ�ȵ�ϡ���ᣬ������ȫ�ܽ⣬�ֵõ���״����1.12 L NO������Ӧ�����Һ�м���KSCN��Һ����Һ����죬������˵����ȷ���ǡ�(����)
A.ԭ�������ͭ���������ʵ�����Ϊ0.075 mol
B.��Ӧǰϡ��������ʵ���Ũ��������
C.�����������������Һ���ټ�������ϡ���ᣬ���ɵõ���״����1.12 L NO
D.��һ����100 mLϡ���ᷴӦ��ʣ���4.8 g����Ϊͭ����
���𰸡�A
��������
��һ�μ�����ʱ������ʣ�࣬���۲μӷ�Ӧ��������������ͭ���ܽ��4.2�˽�������������+2�ۣ�3Fe����3Cu��+8HNO3=3Fe(NO3)2[��3Cu(NO3)2]+2NO��+4H2O�����������ʵ���Ϊ![]() ��
��![]() =0.075mol �����һ���ܽ�Ľ���Ħ������Ϊ56g��mol-1�����Ե�һ���ܽ���������ڶ��μ���������Һ����ʹKSCN��Һ��죬�ܽ��4.8�˽���Ҳһ����������+2�ۣ�ͬ���ɵ������ʵ���Ϊ0.075mol������Ħ������Ϊ64g��mol-1����ͭ��
=0.075mol �����һ���ܽ�Ľ���Ħ������Ϊ56g��mol-1�����Ե�һ���ܽ���������ڶ��μ���������Һ����ʹKSCN��Һ��죬�ܽ��4.8�˽���Ҳһ����������+2�ۣ�ͬ���ɵ������ʵ���Ϊ0.075mol������Ħ������Ϊ64g��mol-1����ͭ��
A. ���ݷ�������һ���ܽ����0.075mol�����ڶ����ܽ����0.075molͭ��ԭ�������ͭ���������ʵ�����Ϊ0.075 mol����A��ȷ��
B. ���ݵ�Ԫ���غ㣬��Ӧǰϡ��������ʵ���Ũ��Ϊ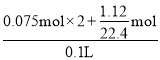 =2mol/L����B����
=2mol/L����B����
C. �����������������Һ���ټ�������ϡ���ᣬ�������ӱ�����Ϊ�����ӣ���ӦΪ3Fe2++4H++NO3-=3Fe3++NO��+2H2O������NO�����ʵ�����![]() ����״���µ����Ϊ0.56 L NO����C����
����״���µ����Ϊ0.56 L NO����C����
D. �������Ϸ�������һ����100 mLϡ���ᷴӦ��ʣ���4.8 g����Ϊͭ������������D����
ѡA��

 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�