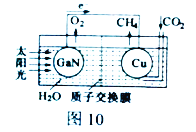
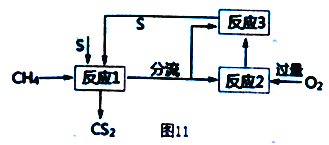
��Ŀ����
����Ŀ��̼����(MnCO3) ������ĸ�����ǿ���Բ��ϣ�Ҳ���Ʊ�Mn2O3��MnO2���̵����������Ҫԭ�ϣ��㷺���ڵ��ӡ�������ҽҩ����ҵ��
(1) ��ҵ���Ʊ�����ʽΪ��MnSO4+2NH4HCO3=MnCO3 ��+ (NH4)2SO4+CO2 ��+H2O����Ӧ��ͨ��������Թ�����NH4HCO3���ҿ�����Һ��pHΪ6.8~7.4�������Թ�����NH4HCO3��Ŀ����_____��
(2) ʵ����ģ�ҵ���������Ʊ�������װ����ͼ4��ʾ��

��ʯ�����������________��
����Ӧ�����У�ΪʹSO2������ת����ȫ����ͨ��SO2��N2����һ�������ı��ҺͶ�ϵ������£��ɲ�ȡ�ĺ�����ʩ��________��
(3) MnCO3 �ڿ����м�����ת��Ϊ��ͬ��̬���̵���������������������¶ȵı仯��ͼ5 ��A��B��C��D ����ʾ����300��ʱ��ʣ���������Ԫ������Ԫ�ص����ʵ���֮�ȼ�n(Mn) :n(O)Ϊ_______�� ͼ�е�D ��Ӧ����ijɷ�Ϊ______ (�ѧʽ)��

���𰸡� ʹMnSO4��ַ�Ӧ�����MnSO4����������NH4HCO3�ֽ� ���ն����SO2�����ٶԻ�������Ⱦ ����ͨ����������� 1:2 Mn3O4��MnO
��������������������⿼�鷴Ӧ�����Ŀ��ƣ���SO2�йص�ʵ�飬����ͼ����ļ��㡣
��1�������Թ�����NH4HCO3����ʹMnSO4��ַ�Ӧ�����MnSO4�������ʣ�NH4HCO3�ֽ�������NH4HCO3Ҫ�Թ�����
��2��������װ��ͼ����������ƿ��SO2��MnO2��Ӧ�Ƶ�MnSO4������SO2��Ⱦ��������ʯ�������ն����SO2�����ٶԻ�������Ⱦ����ӦΪSO2+Ca��OH��2=CaSO3+H2O��
����Ӧ�����У�ΪʹSO2������ת����ȫ����ͨ��SO2��N2����һ�������ı��ҺͶ�ϵ������£��ɲ�ȡ�ĺ�����ʩ��������ͨ��������ʹ���������MnO2������ã��ʵ������¶���
��3����MnCO3���ʵ���Ϊ1mol��m��MnCO3��=1mol![]() 115g/mol=115g������n��Mn��=1mol��300����ʣ����������Ϊ115g
115g/mol=115g������n��Mn��=1mol��300����ʣ����������Ϊ115g![]() 75.65%=87g������m��O��=87g-55g=32g��n��O��=32g
75.65%=87g������m��O��=87g-55g=32g��n��O��=32g![]() 16g/mol=2mol��n��Mn����n��O��=1:2����ͼ�ɼ�D�������B���C��Ĺ����϶��ɣ�B�㣬ʣ����������Ϊ115g
16g/mol=2mol��n��Mn����n��O��=1:2����ͼ�ɼ�D�������B���C��Ĺ����϶��ɣ�B�㣬ʣ����������Ϊ115g![]() 66.38%=76.34g������n��O��=
66.38%=76.34g������n��O��=![]() =1.334mol��n��Mn����n��O��=1mol��1.334mol=3:4��B�����ΪMn3O4��C�㣬ʣ����������Ϊ115g
=1.334mol��n��Mn����n��O��=1mol��1.334mol=3:4��B�����ΪMn3O4��C�㣬ʣ����������Ϊ115g![]() 61.74%=71g������n��O��=
61.74%=71g������n��O��=![]() =1mol��n��Mn����n��O��=1mol��1mol=1:1��C�����ΪMnO��D���Ӧ����ɷ�ΪMn3O4��MnO��
=1mol��n��Mn����n��O��=1mol��1mol=1:1��C�����ΪMnO��D���Ӧ����ɷ�ΪMn3O4��MnO��

 ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д�
ӥ�ɽ̸��νӽ̲ĺӱ�����������ϵ�д�