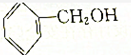��Ŀ����
18�� Ŀǰ��ҵ����һ����CO2������ȼ�ϼ״��ķ��������Խ�CO2���Ϊ����
Ŀǰ��ҵ����һ����CO2������ȼ�ϼ״��ķ��������Խ�CO2���Ϊ������1����֪�ڳ��³�ѹ�£�
��2CH3OH��l��+3O2��g���T2CO2��g��+4H2O��g����H=1275.6kJ•mol-1
��2CO��g��+O2��g���T2CO2��g����H=566kJ•mol-1
��H2O��l��=H2O��g����H=+44kJ•mol-1
д���״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽCH3OH��l��+O2��g��=CO��g��+2H2O��l����H=_442.8kJ•mol-1
��2���״���ˮ�ʻ����һ������Ⱦ����һ�ֵ绯ѧ��������������Ⱦ��ʵ��������ͼװ��ģ��ù��̣���ԭ���ǣ�ͨ���Co2+��������Co3+��Ȼ����Co3+���������Ѽ״�������CO2�����ƣ�Co3+�Ļ�ԭ������Co2+����
��д�������缫��ӦʽCo2+-e-=Co3+��
��д�����Ƽ״������ӷ���ʽ6Co3++CH3OH+H2O=CO2��+6Co2++6H+��
��3������ͬ����CO��g����H2O��g���ֱ�ͨ�����Ϊ2L�ĺ����ܱ������У����з�Ӧ��CO��g��+H2O��g��?CO2��g��+H2��g�����õ������������ݣ�
| ʵ���� | �¶�/�� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ������ʱ��/min | |
| H2O | CO | CO2 | |||
| 1 | 650 | 2 | 4 | 1.6 | 5 |
| 2 | 900 | 1 | 2 | 0.4 | 3 |
| 3 | 900 | 1 | 2 | 0.4 | 1 |
��ʵ��1�У���v��H2����ʾ��ƽ����Ӧ����Ϊ0.16mol•��L•min��-1��
��900��ʱ����CO��g����H2O��g����CO2��g����H2��g�������ʵ����ֱ���0.8mol��1.0mol��0.6mol��0.8mol�ֱ��������������ʱ��Ӧ��v��������v���棩���������������=��֮һ����
��ʵ��3��ʵ��2��ȣ��ı������������ʹ���˴�����������ѹǿ��
���� ��1�������Ȼ�ѧ����ʽ��˹���ɼ���õ���
��2����ͨ���Co2+������Co3+������������ʧ���ӷ���������Ӧ���缫��ӦΪCo2+-e-=Co3+��
����Co3+����������ˮ�еļ״�������CO2����������������ԭΪCo2+��ԭ���غ������غ��֪����ԭ����H+����ƽ��дΪ��6Co3++CH3OH+H2O=CO2��+6Co2++6H+��
��3��������ת����������������ʼ���Ĺ�ϵ���㲢�жϷ�Ӧ�ȣ�
�����û�ѧ��Ӧ���ʵļ��㹫ʽ���㣻
�۾�Qc��K�Ĵ�С�жϷ�Ӧ����
��ʵ��3��ʵ��2��ȣ��¶���ͬ��Ũ����ͬ����ʵ��3�ﵽƽ������ʱ���٣���Ӧ���ʸ���ƽ��״̬һ����
��� �⣺��1����2CH3OH��l��+3O2��g��=2CO2��g��+4H2O��g����H=_1275.6kJ•mol-1
��2CO��g��+O2��g��=2CO2��g����H=_566.0kJ•mol-1
��H2O��l��=H2O��g����H=+44.0kJ•mol-1
�����Ȼ�ѧ����ʽ��˹���ɢ�-��-�ۡ�4�õ�CH3OH��l��+O2��g��=CO��g��+2H2O��l����H=_442.8 kJ•mol-1��
�ʴ�Ϊ��CH3OH��l��+O2��g��=CO��g��+2H2O��l����H=_442.8 kJ•mol-1��
��2����ͨ���Co2+������Co3+������������ʧ���ӷ���������Ӧ���缫��ӦΪCo2+-e-=Co3+��
�ʴ�Ϊ��Co2+-e-=Co3+��
����Co3+����������ˮ�еļ״�������CO2����������������ԭΪCo2+�����ԭ���غ������غ��֪����ԭ����H+����ƽ��д���ӷ���ʽΪ��6Co3++CH3OH+H2O=CO2��+6Co2++6H+��
�ʴ�Ϊ��6Co3++CH3OH+H2O=CO2��+6Co2++6H+��
��3����ʵ��1��CO��ת����Ϊ$\frac{4mol-2.4mol}{2mol}$��100%=40%��ʵ��2��CO��ת����Ϊ$\frac{2mol-1.6mol}{2mol}$��100%=20%����ʵ��1��ת���ʴ���ʵ��2��
��˵���¶�����ƽ�����淴Ӧ�����ƶ����÷�Ӧ���ȣ��ʴ�Ϊ���ţ�
��v��H2��=v��CO2��=$\frac{\frac{1.6mol}{2L}}{5min}$=0.16mol/��L•min��=v��H2�����ʴ�Ϊ��0.16mol•��L•min��-1��
���ڷ�ӦCO��g��+H2O��g��?CO2��g��+H2��g���У�2L�����п�ʼ����1molH2O��2molCO��ƽ��ʱ���������������̼���ʵ�����ͬΪ0.4mol��������ƽ��Ũ��Ϊc��H2O��=0.3mol/L��c��CO��=0.8mol/L��c��H2��=c��CO2��=0.2mol/L��K=$\frac{0.2��0.2}{0.3��0.8}$=$\frac{1}{6}$��Qc=$\frac{0.4��0.3}{0.4��0.5}$=$\frac{3}{5}$��K��ƽ�������ƶ���v��������v���棩�ʴ�Ϊ������
��ʵ��3��ʵ��2��ȣ��¶���ͬ��Ũ����ͬ����ʵ��3�ﵽƽ������ʱ���٣���Ӧ���ʸ���ƽ��״̬һ����Ӧ��ʹ���˴��������ڷ�Ӧǰ������������䣬��������ѹǿ��ƽ��Ҳ���ƶ���Ҳ����Ϊѹǿ������
�ʴ�Ϊ��ʹ���˴�����������ѹǿ��
���� ���⿼���˸�˹���ɵ�Ӧ�á����ԭ�������͵缫��Ӧ��д��������ѧƽ��ļ��㣬��Ŀ�Ѷ��еȣ�����ʱע�����Ӱ��ƽ���ƶ��������Լ�ƽ�ⳣ�����йؼ��㣮

| A�� | ������ͭ��Һ�м���NaHS��Һ��Cu2++HS-=CuS��+H+ | |
| B�� | �ô����ȥˮ���е�̼��ƣ�CaCO3+2H+=Ca2++H2O+CO2�� | |
| C�� | ��ǿ����Һ���չ�ҵ��ȡ����β����NO+NO2+2OH-=2NO3-+H2O | |
| D�� | ������SO2����ͨ�백ˮ�У�SO2+NH3•H2O=NH4++HSO3- |
| A�� | ��Ũ�����Ũ����ֱ���¶���ڿ����У�Ũ�Ⱦ��ή�� | |
| B�� | �ڳ����£�Ũ�����Ũ���������ͭ���ҷ�Ӧ | |
| C�� | ϡ�����ϡ����ֱ��������Ӧʱ��S��NԪ�صĻ��ϼ۶��ᷢ���仯 | |
| D�� | ��ΪŨ�����Ũ���ᶼ��������Ӧ�����Գ����¶��߶���������������ʢװ |
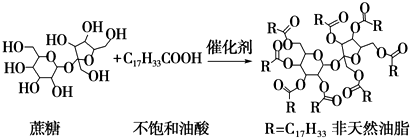
����˵����ȷ���ǣ�������
| A�� | ������Ҳ�Ǹ�֬����ĸ�������������֬������ | |
| B�� | �÷���Ȼ��֬������������Һ���ȣ���ˮ����ﲻ����ˮ��Ӧ | |
| C�� | ����Ȼ��֬Ϊ�߷��ӻ����� | |
| D�� | ����������ϡ�����������ˮ�⣬���տ����������л������� |
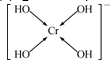
| A�� | �ڷ�Ӧ��SnԪ�ر���ԭ��AsԪ�ر����� | |
| B�� | ��������SnCl2����������ΪSnCl62- | |
| C�� | ������MΪOH- | |
| D�� | ÿ����7.5gAs����ԭ��ʧȥ0.3mol���� |
�ٳ����£�20mL��Һ�к�A��B��0.001mol��
�ڳ����£�100mL��Һ�к�A��B��0.01mol��
�۳����£���10mL��A��B��0.0005mol����Һ���ټ�������ˮ30ml��
�ܳ����£�100mL��Һ�к�A 0.01mol��B 0.005mol��
| A�� | �٢ڢۢ� | B�� | �ܢۢڢ� | C�� | �ڢܢ٢� | D�� | �ڢ٢ܢ� |
| A�� | ������ | B�� | ��Ե�� | C�� | ������ | D�� | �۵�� |
 ��������λ�����ü��ű�ʾ��
��������λ�����ü��ű�ʾ��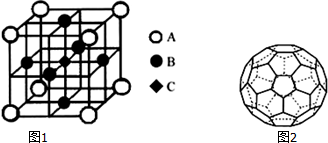
 b��
b��