��Ŀ����
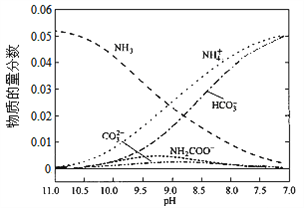
����Ŀ����ͼ��ϸ����3�ֻ����ﺬ��������ͼ��ͼ2�ǻ�ϸ����Ԫ�غ���������ͼ������˵���в���ȷ���ǣ� ��
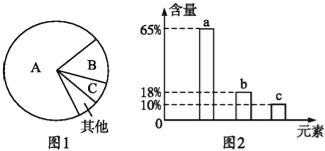
A. ͼ1��A��B��C�ֱ���ˮ�������ʡ�֬�ʣ�ͼ2��a��b��c����Ԫ�����α�ʾ̼���⡢��
B. ��ͼ1��ʾ����ϸ������B��������ж����ԣ���غ�ͼ2�е�a��b��c
C. ��ͼ1��ʾ����ϸ������A�в���ͼ2�е�b
D. ͼ1���Ա�ʾ����ϸ����ȫ��ˮ���ﺬ��������ͼ������ʱ��������Ԫ��Ϊͼ2�е�b
���𰸡�A
��������
�������ϸ�����ص�Ԫ�أ�������������Ԫ������������̼���⡢����A������ͼ1�DZ�ʾ����ϸ������B�������ǵ����ʣ����ж����ԣ���C��H��O��N��Ԫ����ɣ�B��ȷ����ͼ1��ʾ����ϸ������A��ʾˮ��C��ȷ�����ϸ�����ص�Ԫ�أ�������������Ԫ��������̼�����������⣬D��ȷ��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ