��Ŀ����
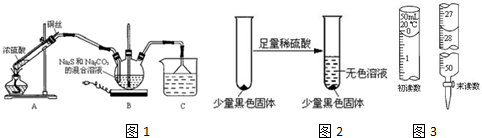
16����������ƣ�Na2S2O3���ڹ�ҵ������ҽҩ����ҵ�б��㷺Ӧ�ã���ҵ�ձ�ʹ��Na2SO3����ǣ�S������õ���װ����ͼ1����֪��Na2S2O3+H2SO4�TNa2SO4+SO2��+S��+H2O��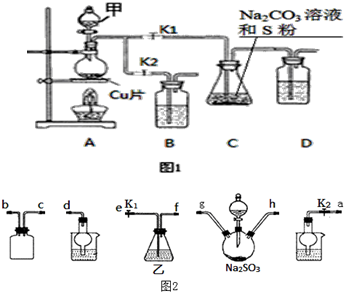
��1������1����K1���ر�K2����Բ����ƿ�м��������ײ����ȣ����Լ���ΪŨ���ᣮ
��2������2��ʼ�ձ���C����Һ�ʼ��ԣ���Ӧһ��ʱ�����۵������٣���K2���ر�K1��ֹͣ���ȣ�
��C����Һ�뱣�ֳʼ��ԣ��������ԣ���CO32-+2H+=H2O+CO2����S2O32-+2H+=H2O+SO2��+S�����������ӷ���ʽ��ʾ��
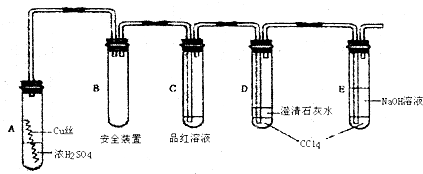
��װ��B��D������������SO2����ֹ��Ⱦ��
��3������3����C�����û��������ᴿ��ò�Ʒ��
��4�����÷�Ӧ2Na2S+Na2CO3+4SO2=3Na2S2O3+CO2Ҳ���Ʊ�Na2S2O3������������ͼ2���������������Ӹ��������ӿ�˳��Ϊ��a��g��h��b��c��e��f��d��
��5��װ����ʢװ���Լ���Na2CO3��Na2S�Ļ����Һ��
��6��Na2S2O3��ԭ�Խ�ǿ����ҵ�ϳ�������ȥ��Һ�в�����Cl2���÷�Ӧ�����ӷ���ʽΪS2O32-+4Cl2+5H2O=2SO42-+8Cl-+10H+��
��7������Ƽ�ʵ�鷽����֤������������Cl2����ԭ����Cl-ȡ������Ӧ�����Һ���Թ��У�����Ba��NO3��2��Һ�����ٲ�����������ȡ�ϲ���Һ������˺�ȡ��Һ�����μ�AgNO3��Һ����������ɫ��������˵��Cl2����ԭΪ��Cl-��
���� ��1����ʵ��װ�ÿ�֪A�����Ʊ���������BΪβ������װ�ã�C�з�Ӧ����Na2S2O3��DΪβ������װ�ã��Դ˽��
��2����̼��������������������Ӳ����棻
���������������������Ӧ��
��4��װ��gh��Ũ������������Ʒ�Ӧ���ɶ������������װ�ã�װ��bc�ǰ�ȫƿ����������¶����������ֹ���������ã�������������Ⱦ������ʵ����������ŷŵ������У�װ��d�еķ�Ӧ�������ȹرշ�Һ©���������������IJ����ǹر�K1��K2��
��5��װ�����з�����Ӧ2Na2S+Na2CO3+4SO2=3Na2S2O3+CO2��
��6��Na2S2O3��������Ӧ�����������
��7���������ᱵ�����������Ȼ����������ӣ�
��� �⣺��1��A�����Ʊ���������ӦΪŨ�����ͭ�ڼ��������·�Ӧ�����Լ���ΪŨ���ᣬ�ʴ�Ϊ��Ũ���
��2����̼��������������������Ӳ����棬���ӷ���ʽ��CO32-+2H+=H2O+CO2����S2O32-+2H+=H2O+SO2��+S����
�ڣ��ʴ�Ϊ��CO32-+2H+=H2O+CO2����S2O32-+2H+=H2O+SO2��+S����
��װ��B��D��Ӧʢ������������Һ����������SO2����ֹ��Ⱦ�������ʴ�Ϊ������SO2����ֹ��Ⱦ��
��4��װ��gh��Ũ������������Ʒ�Ӧ���ɶ������������װ�ã�װ��bc�ǰ�ȫƿ����������¶����������ֹ���������ã�������������Ⱦ������ʵ����������ŷŵ������У�װ��d�еķ�Ӧ�������ȹرշ�Һ©���������������IJ����ǹر�K1��K2���ӿ�˳��Ϊ��a��g��h��b��c��e��f��d���ʴ�Ϊ��a��b��c��e��f��
��5��װ�����з�����Ӧ2Na2S+Na2CO3+4SO2=3Na2S2O3+CO2��ʢװ���Լ���Na2CO3��Na2S�Ļ����Һ���ʴ�Ϊ��Na2CO3��Na2S�Ļ����Һ��
��6��Na2S2O3��������Ӧ��������������ӷ���ʽΪ��S2O32-+4Cl2+5H2O=2SO42-+8Cl-+10H+���ʴ�Ϊ��S2O32-+4Cl2+5H2O=2SO42-+8Cl-+10H+��
��7���������ᱵ�����������Ȼ����������ӣ�ȡ������Ӧ�����Һ���Թ��У�����Ba��NO3��2��Һ�����ٲ�����������ȡ�ϲ���Һ������˺�ȡ��Һ�����μ�AgNO3��Һ����������ɫ��������˵��Cl2����ԭΪ��Cl-���ʴ�Ϊ��ȡ������Ӧ�����Һ���Թ��У�����Ba��NO3��2��Һ�����ٲ�����������ȡ�ϲ���Һ������˺�ȡ��Һ�����μ�AgNO3��Һ����������ɫ��������˵��Cl2����ԭΪ��Cl-��
���� ���⿼�����ʵ��Ʊ��������е��Ѷ�����Ŀ��飬�����ۺ���ǿ�����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ����ּ�ڿ���ѧ��������û���֪ʶ���ʵ�����������������������ѧ���������������淶�Ͻ���ʵ��������������ѧ����ѧ��������

 �����ҵ���������ϵ�д�
�����ҵ���������ϵ�д�| A�� | Na��Mg��Al��Zn��Fe | B�� | Al��Mg��Na��Fe��Zn | C�� | Mg��Al��Na��Zn��Fe | D�� | Zn��Fe Na��Mg��Al�� |
| A�� | 19�� | B�� | 10�� | C�� | 16�� | D�� | 12�� |
| A�� | �����ʵ�����Ba��OH��2��NH4HSO4��ϡ��Һ�з�Ӧ��Ba2++2OH-+2H++SO42-=BaSO4��+2H2O | |
| B�� | �����������������������Һ��Ӧ��Fe��OH��3+3H+=Fe3++3H2O | |
| C�� | Ca��HCO3��2��Һ������NaOH��Һ��Ӧ��Ca2++2HCO3-+2OH-=CaCO3��+CO32-+2H2O | |
| D�� | ��100mL 1mol/L FeBr2��Һ��ͨ��0.25mol Cl2��2Fe2++2Br-+2Cl2=2Fe3++Br2+4Cl- |
| A�� | NH3��ˮ��Һ���Ե��磬˵��NH3�ǵ���� | |
| B�� | Na2O2ת��ΪNa2CO3ʱһ����Ҫ���뻹ԭ������ʵ�� | |
| C�� | Fe2+��Cl2��SO2�������Ⱦ��������ԣ��־��л�ԭ�� | |
| D�� | ��ɢϵ�з�ɢ�����ӵ�ֱ����Fe��OH��3���壾Fe��OH��3����Һ��FeCl3��Һ |