��Ŀ����
����Ŀ�������£���Ũ��Ϊ0.1 molL��1�����ΪV L�İ�ˮ����μ���һ��Ũ�ȵ����ᣬ��pH�Ʋ���Һ��pH������ļ����������͵ĵζ����ߣ�d��������Һǡ����ȫ��Ӧ������ͼ����Ϣ�ش��������⣺
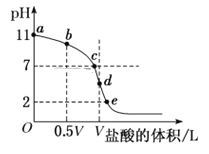
��1�����¶�ʱNH3��H2O�ĵ��볣��K��________��
��2���ζ����������������Ũ��________molL��1��
��3���Ƚ�b��c��d����ʱ����Һ�У�ˮ����̶�����Ϊ___________��(����ĸ)
��4���ζ�ʱ����b�㵽c��Ĺ����У����и�ѡ������ֵ������___(����ĸ����ͬ)��
A�� c(H��)/c(OH��) B��c(H��)��c(NH3��H2O)/c(NH4+)
C��c(NH4+)��c(OH��)/c(NH3��H2O) D��c(H��)��c(OH��)
��5���������ϵζ������ж�����˵����ȷ����______(��Һ��NԪ��ֻ����NH4+��NH3��H2O������ʽ)��
A����b��ʾ��Һ�У�c(NH4+)��c(H��)��c(OH��)��c(NH3��H2O)
B����c��ʾ��Һ�У�c(Cl��)��c(NH3��H2O)��c(NH4+)
C����d��ʾ��Һ�У�c(Cl��)>c(H��)>c(NH4+)>c(OH��)
D���ζ������п����У�c(NH3��H2O)>c(NH4+)>c(OH��)>c(Cl��)>c(H��)
��6��d��֮������������������ͼ���е�e��(������NH4+ˮ���Ӱ��)����e���Ӧ�ĺ�����Ϊ________��
���𰸡�10��5 0.1 d A D 11V/9
��������
��1��һˮ�ϰ���������ʣ���ˮ��Һ�ﲿ�ֵ��룬��ͼ��֪0.1 molL��1��ˮ����Һ��pH=11��c(OH��)Ϊ10��3 molL��1�����ݵ��볣����ʽ���㣻
��2����ͼ���֪����d��ˮ�����������ͬ������ǡ����ȫ��Ӧǡ����ȫ��Ӧ����NH4C1��
��3��c��b��Һ�ж�����һˮ�ϰ���d����Һ�е��������Ȼ�泥�һˮ�ϰ�����ˮ������һˮ�ϰ�Ũ��Խ�������Ƴ̶�Խ���Ȼ�林ٽ�ˮ���룻��d��ˮ�����������ͬ������ǡ����ȫ��Ӧǡ����ȫ��Ӧ����NH4C1��
��4���¶Ȳ��䣬ˮ�����ӻ�������������ʵĵ���ƽ�ⳣ�����䣻
��5����ͼ���֪����b��Ӧ����Һ��NH4C1��NH3H2O���ʵ���֮��Ϊ1��1�Ļ�����Һ�ʼ��ԣ���c ��Һ��pH=7����Һ��c��H+��=c��OH-������NH3H2O�϶࣬�����HCl����ʱ������NH4C1��������Һ��NH3H2OŨ��Զ����NH4C1Ũ�ȣ�
��6���ȼ����������������ټ������������Һ�������
��1��һˮ�ϰ���������ʣ���ˮ��Һ�ﲿ�ֵ��룬��ͼ��֪0.1 molL��1��ˮ����Һ��pH=11��c(OH��)Ϊ10��3 molL��1�����¶�ʱNH3��H2O�ĵ��볣��K��![]() =
=![]() =10��5���ʴ�Ϊ��10��5��
=10��5���ʴ�Ϊ��10��5��
��2���������Ϣ��֪��d��������Һǡ����ȫ��Ӧ�������ʵ���֮�ȵ��ڻ�ѧ������֮�ȿɵ����¹�ϵʽ��0.1 molL��1��V L=c��HCl����V L����c��HCl��=0.1 molL��1���ʴ�Ϊ��0.1��
��3����b��ʱ������Ϊ��ˮ���Ȼ�泥���ˮ�ĵ���̶ȴ����Ȼ�淋�ˮ��̶ȣ���ҺΪ���ԣ���ˮ�ĵ���ƽ�����������ã���c��ʱ������Ϊ��ˮ���Ȼ�泥���ˮ�ĵ���̶ȵ������Ȼ�淋�ˮ��̶ȣ���ҺΪ���ԣ���ˮ�ĵ���ƽ����Ӱ�죬��d��ʱ������Ϊ�Ȼ�泥��Ȼ�立���ˮ�⣬��ҺΪ���ԣ���ˮ�ĵ���ƽ��ٽ�������b��c��d����ʱ����Һ��ˮ����̶��ɴ�С��˳��Ϊd>c>b���ʴ�Ϊ��d��
��4��a�����ŷ�Ӧ�Ľ��У���Һ���Լ�������Һ��������Ũ����������������Ũ�ȼ�С��������Һ��![]() ������ȷ��
������ȷ��
b��NH3��H2O�ĵ��볣��K��![]() ����
����![]() =
=![]() =
=![]() ���¶Ȳ��䣬ˮ�����ӻ�������һˮ�ϰ�����ƽ�ⳣ�������䣬����Һ��
���¶Ȳ��䣬ˮ�����ӻ�������һˮ�ϰ�����ƽ�ⳣ�������䣬����Һ��![]() ���䣬�ʴ���
���䣬�ʴ���
c��NH3��H2O�ĵ��볣��K��![]() ���¶Ȳ��䣬һˮ�ϰ�����ƽ�ⳣ�����䣬�ʴ���
���¶Ȳ��䣬һˮ�ϰ�����ƽ�ⳣ�����䣬�ʴ���
d��ˮ�����ӻ�����Kw= c(H��)��c(OH��)���¶Ȳ��䣬ˮ�����ӻ��������䣬�ʴ���
a��ȷ���ʴ�Ϊ��a��
��5��A����ͼ���֪����b��Ӧ����Һ��NH4C1��NH3H2O���ʵ���֮��Ϊ1��1�Ļ�����Һ�ʼ��ԣ�˵��NH3H2O����̶ȴ���NH4C1��ˮ��̶ȣ��ɵ���غ��֪��c��C1-��+c��OH-��=c��NH4+��+c��H+���������غ�Ϊ��2c��C1-��=c��NH4+��+c��NH3��H2O���������غ�Ϊ��2c��
B����ͼ���֪����c ��Һ��pH=7����Һ��c��H+��=c��OH-�����ɵ���غ��ϵc��C1-��+c��OH-��=c��NH4+��+c��H+����֪����Һ��c��NH4+��=c��C1-�����ʴ���
C����ͼ���֪����d��ˮ�����������ͬ������ǡ����ȫ��Ӧǡ����ȫ��Ӧ����NH4C1��NH4C1����Һ��ˮ����Һʹ��Һ�����ԣ�����Һ������Ũ�ȴ�СΪc��C1-����c��NH4+����c��H+����c��OH-�����ʴ���
D����NH3H2O�϶࣬�����HCl����ʱ������NH4C1��������Һ��NH3H2OŨ��Զ����NH4C1Ũ�ȣ����ܳ���c��NH3H2O����c��NH4+����c��OH-����c��Cl-����c��H+��������ȷ��
D��ȷ���ʴ�Ϊ��D��
��6������������ΪXL�������������Ϊ0.1mol/L��XL-0.1Vmol/L��VL����c��H+��=![]() =10-2mol/L����ã�X=
=10-2mol/L����ã�X=![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��