��Ŀ����
�ɼ������ӻ�������ɵĻ����������������е������֣�K����NH��Ba2����Al3����Fe3����Cl����SO42-��CO32-�����û��������ˮ��ó�����Һ����ȡ3��100 mL����Һ�ֱ��������ʵ�飺
�Իش��������⣺
(1)����ʵ��1��CO32-�Ƿ���ڵ��ж���________��(�һ�����ڡ���һ�������ڡ�����ȷ����)������ʵ��1��3�жϻ������һ�������ڵ�������________��
(2)��ȷ����Һ��һ�����ڵ������Ӽ������ʵ���Ũ��(�ɲ�����)��
| ��� | ʵ������ | ʵ���� |
| 1 | �ӹ������� | ��������� |
| 2 | ������NaOH��Һ������ | �ռ�������1.12 L(������ɱ�״���µ����)�����к��ɫ�������ɣ�����������ϴ�ӡ�������գ����ص�1.60 g���塣 |
| 3 | ������BaCl2��Һʱ�������ó�������ϴ�ӡ�������� | ��һ�γ�������Ϊ2.33 g |
�Իش��������⣺
(1)����ʵ��1��CO32-�Ƿ���ڵ��ж���________��(�һ�����ڡ���һ�������ڡ�����ȷ����)������ʵ��1��3�жϻ������һ�������ڵ�������________��
(2)��ȷ����Һ��һ�����ڵ������Ӽ������ʵ���Ũ��(�ɲ�����)��
| �����ӷ��� | ���ʵ���Ũ��(mol��L��1) |
| | |
| | |
| | |
(1)һ�������ڣ�Ba2����(2)���±���
| �����ӷ��� | ���ʵ���Ũ��(mol��L��1) |
| Cl�� | 0.9 |
| SO42- | 0.1 |
����ʵ��1��ȷ��һ������CO32-������NaOH�ܲ������壬˵������NH�������ʵ���Ϊ0.05 mol���������ɫ����˵����Fe3���������ʵ���Ϊ0.02 mol��������BaCl2��Һ�������������������ɼ��������ʵ����ֱ�Ϊ0.01 mol�����ݵ���غ��֪��K��һ�����ڡ�

��ϰ��ϵ�д�
�����Ŀ

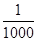 ����������CNO������
�������Σ���CNO������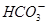 ��ȫת��ΪCaCO3
��ȫת��ΪCaCO3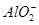 ��ʽ����
��ʽ����