��Ŀ����
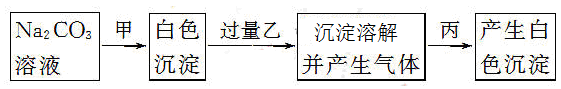
ijУͬѧΪ̽��Br2��I2��Fe3����������ǿ��������������ʵ�顣
ʵ��٣�ȡ����KI��Һ���Թ��У��ȼ�����ˮ�����ټ���CCl4�����ã��۲쵽�²�Һ����Ϻ�ɫ��
ʵ��ڣ�ȡ����FeSO4��Һ���Թ��У��ȼ�����ˮ�����ټ����μ�����KSCN��Һ�����۲쵽��Һ�ʺ�ɫ��
(1)д�����ӷ���ʽ��
ʵ��٣�__________________________________________________________��
ʵ��ڣ�__________________________________________________________��
(2)����������ʵ�飬�����ʵ������Կ��Եó�����ȷ������________��
(3)��֪Fe3����������ǿ��I2������������Լ���ѡ�������Լ������һ��ʵ�����֤����(��ʾ����д��ʵ�鲽�衢������)
��FeCl3��Һ���ڵ�ˮ����KI��Һ����ϡH2SO4���ݵ�����Һ
__________________________________________________________________
__________________________________________________________________
ʵ��٣�ȡ����KI��Һ���Թ��У��ȼ�����ˮ�����ټ���CCl4�����ã��۲쵽�²�Һ����Ϻ�ɫ��
ʵ��ڣ�ȡ����FeSO4��Һ���Թ��У��ȼ�����ˮ�����ټ����μ�����KSCN��Һ�����۲쵽��Һ�ʺ�ɫ��
(1)д�����ӷ���ʽ��
ʵ��٣�__________________________________________________________��
ʵ��ڣ�__________________________________________________________��
(2)����������ʵ�飬�����ʵ������Կ��Եó�����ȷ������________��
| A��Br2>I2 | B��Fe3��>Br2 |
| C��Br2>Fe3�� | D��I��>Br�� |
��FeCl3��Һ���ڵ�ˮ����KI��Һ����ϡH2SO4���ݵ�����Һ
__________________________________________________________________
__________________________________________________________________
(1)��2I����Br2=I2��2Br������2Fe2����Br2=2Fe3����2Br����(2)AC
(3)ȡ����FeCl3��Һ�ڽྻ�Թ��У����μ���KI��Һ�͵�����Һ��������Һ������֤��Fe3����������ǿ��I2��
(3)ȡ����FeCl3��Һ�ڽྻ�Թ��У����μ���KI��Һ�͵�����Һ��������Һ������֤��Fe3����������ǿ��I2��
��ʵ���֪������Fe3��������Ԫ�ػ��ϼ����ߣ���ô��Ԫ�صĻ��ϼ۱�Ȼ��������Br������ӦΪ2Fe2����Br2=2Fe3����2Br������ʵ���֪������I2����ӦΪ2I����Br2=I2��2Br������������Ӧ�У�Br2����������Fe3����I2�������������������Br2>I2��Br2>Fe3����Fe3����������ǿ��I2�����Ը������·�Ӧ��ƣ�2Fe3����2I��=2Fe2����I2��

��ϰ��ϵ�д�
�����Ŀ

 2Cu��O2����4H��
2Cu��O2����4H��
 Cl2
Cl2