��Ŀ����
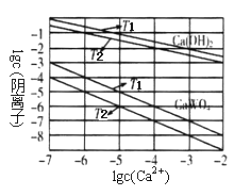
����Ŀ��һ������CO2��������̼������ɱ�ĺ�ѹ�ܱ������з�Ӧ��C(s)+CO2(g)![]() 2CO(g)��ƽ��ʱ����ϵ����������������¶ȵĹ�ϵ����ͼ��ʾ��
2CO(g)��ƽ��ʱ����ϵ����������������¶ȵĹ�ϵ����ͼ��ʾ��

��֪�������ѹ��P����=������ѹ��P�������������������˵����ȷ����
A. 550��ʱ��������������壬������ ����С��ƽ�ⲻ�ƶ�
B. 650��ʱ����Ӧ��ƽ���CO2��ת����Ϊ25.0%
C. T��ʱ��������������CO2��CO��ƽ�����淴Ӧ�����ƶ�
D. 925��ʱ����ƽ���ѹ����ƽ��Ũ�ȱ�ʾ�Ļ�ѧƽ�ⳣ��KP=24.0P��
���𰸡�B
��������
A�����ڷ�Ӧ������ɱ�ĺ�ѹ�ܱ������н��У���550��ʱ��������������壬�������ݻ�����ʹ��Ӧ������Ũ�ȼ�С����������� ����С�����ڸ÷�Ӧ�������������ķ�Ӧ����Сѹǿ����ѧƽ�������������������Ӧ�����ƶ���A����B������ͼ���֪��650��ʱ����Ӧ��ƽ���CO�����������40%����CO2�����������60%������ƽ��ʱ�����ʵ�����1 mol����Ӧ����CO 0.4 mol�����к���CO2 0.6 mol����Ӧ����0.4 molCO����CO2�����ʵ�����0.2 mol�����CO2ת����Ϊ0.2 mol��(0��6 mol+0.2 mol)��100%=25.0%��B��ȷ��C��T��ʱ��ƽ��ʱCO2��CO�������������50%��������������CO2��CO����ѧƽ�ⲻ�ƶ���C����D��925��ʱ����ƽ���ѹ����ƽ��Ũ�ȱ�ʾ�Ļ�ѧƽ�ⳣ��KP=![]() ��D����ѡB��
��D����ѡB��

 ��ҵ����ϵ�д�
��ҵ����ϵ�д�