��Ŀ����
����Ŀ���������ȣ�ClO2����Ϊһ�ָ�Чǿ���������ѱ����Ϲ�����������֯(WHO)��ΪAI����ȫ�������������¶�������Ϊ����ɫ���ٻ�ɫ���壬���ʷdz����ȶ����¶ȹ��ߣ��������ȵ�ˮ��Һ�п��ܱ�ը�����Ʊ������У�
��1������һ���������ƣ�NaClO3���������ữ��H2O2��Ӧ����ClO2����д���÷�Ӧ�Ļ�ѧ����ʽ___________________________________________��
��2����������������������Ũ���ᣬ���ɶ������ȵ�ͬʱ����������������д���÷�Ӧ�Ļ�ѧ����ʽ_____________________________________��
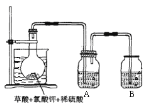
��3����������
����ͼ,����ƿ���ȷ���һ������KClO3�Ͳ���(H2C2O4)��
Ȼ���ټ���������ϡ���ᣬˮԡ���ȡ���Ӧ���������
ClO2��CO2��һ����ʽ�Σ��÷�Ӧ�Ļ�ѧ����ʽΪ��__________________________________��
���������뻹ԭ��������ʵ���֮��Ϊ____________��
�ڿ���ˮԡ�¶���60��80��֮���Ŀ����_______________________��
ͼʾװ����ȱ�ٵ�һ�ֱ���IJ���������____________��
��Aװ���������ղ����Ķ������ȣ��������ʢ��________��������ţ���60 �����ˮ �ڱ�ˮ �۱���ʳ��ˮ
�ܽ���������������Һ���뵽������Һ�У�������Һ�м��������Ȼ�����Һ���а�ɫ�������ɡ���д������������Һ��������Һ��Ӧ�����ӷ���ʽ_______________________��
���𰸡�2NaClO3+H2SO4+H2O2==2ClO2 + Na2SO4+O2 +2H2O2NaClO3+4HCl=2NaCl+Cl2��+2ClO2��+2H2O![]() 1:1ʹ��Ӧ�������У�����ֹ�¶ȹ�������ը�¶ȼƢ�5H2S+8ClO2+4H2O=5SO42��+8Cl��+18H+
1:1ʹ��Ӧ�������У�����ֹ�¶ȹ�������ը�¶ȼƢ�5H2S+8ClO2+4H2O=5SO42��+8Cl��+18H+
��������
��1��������������������H2O2����ԭ�����������ữ��Ӧ����ClO2��Na2SO4��O2 ��H2O���÷�Ӧ�Ļ�ѧ����ʽΪ2NaClO3+H2SO4+H2O2=2ClO2+Na2SO4+O2 ![]() +2H2O ���𰸣�2NaClO3+H2SO4+H2O2==2ClO2+Na2SO4+O2
+2H2O ���𰸣�2NaClO3+H2SO4+H2O2==2ClO2+Na2SO4+O2 ![]() +2H2O��
+2H2O��
��2������������������Ũ��������ԭ�������ɶ������ȵ�ͬʱ���������������÷�Ӧ�Ļ�ѧ����ʽ2NaClO3+4HCl=2NaCl+Cl2��+2ClO2��+2H2O���𰸣�2NaClO3+4HCl=2NaCl+Cl2��+2ClO2��+2H2O��
��3������ͼ,����ƿ���ȷ���һ������KClO3�Ͳ���(H2C2O4)��Ȼ���ټ���������ϡ���ᣬˮԡ���ȡ���Ӧ����ClO2��CO2��һ����ʽ�Σ��÷�Ӧ�Ļ�ѧ����ʽΪ��2KClO3 +H2C2O4+ 2H2SO4![]() 2ClO2+2H2O+2CO2+2KHSO4����������ΪCO2����ԭ����ΪClO2�����������뻹ԭ��������ʵ���֮��Ϊ1:1���𰸣�
2ClO2+2H2O+2CO2+2KHSO4����������ΪCO2����ԭ����ΪClO2�����������뻹ԭ��������ʵ���֮��Ϊ1:1���𰸣�![]() 1:1 ��
1:1 ��
����Ϊ�����¶�������Ϊ����ɫ���ٻ�ɫ���壬���ʷdz����ȶ����¶ȹ��ߣ��������ȵ�ˮ��Һ�п��ܱ�ը�����Կ���ˮԡ�¶���60��80��֮���Ŀ����ʹ��Ӧ�������У�����ֹ�¶ȹ�������ը������ˮԡ���ȵ��¶ȵ���ˮ�ķе㣬��Ҫ���¶ȼƿ���ˮ���¶ȣ���ͼʾװ����ȱ�ٵ�һ�ֱ���IJ����������¶ȼ����𰸣�ʹ��Ӧ�������У�����ֹ�¶ȹ�������ը���¶ȼ���
����Ϊ�����¶�������Ϊ����ɫ���ٻ�ɫ���壬���ʷdz����ȶ����¶ȹ��ߣ��������ȵ�ˮ��Һ�п��ܱ�ը������Aװ���������ղ����Ķ������ȣ��������ʢ�ű�ˮ���𰸣��ڡ�
������������������Һ���뵽������Һ�У�������Һ�м��������Ȼ�����Һ���а�ɫ�����������������ȿ��Խ���������Ϊ��������ӡ����Զ���������Һ��������Һ��Ӧ�����ӷ���ʽ5H2S+8ClO2+4H2O=5SO42��+8Cl��+18H+���𰸣�5H2S+8ClO2+4H2O=5SO42-+8Cl-+18H+��

 ��ѧ��ʦ����ϵ�д�
��ѧ��ʦ����ϵ�д�����Ŀ���±�����ʾ���ʻ�����Ĵ�����ϵ������Ȧ����
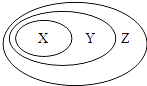
X | Y | Z | |
A | ��Ԫ�� | ����Ԫ�� | ������Ԫ�� |
B | ����� | ������ | ������ |
C | �������� | ��ɢϵ | ���� |
D | �û���Ӧ | ������ԭ��Ӧ | ���ȷ�Ӧ |
A. A B. B C. C D. D
