��Ŀ����
19�� �����³������ؾ����������ṹ�����������Ļ��ϼ۲���Ϊ0�ۣ�����Ϊ-2�ۣ���ͼ��ʾΪ�������ؾ����һ����������������С���ظ���Ԫ����������˵������ȷ���ǣ�������
�����³������ؾ����������ṹ�����������Ļ��ϼ۲���Ϊ0�ۣ�����Ϊ-2�ۣ���ͼ��ʾΪ�������ؾ����һ����������������С���ظ���Ԫ����������˵������ȷ���ǣ�������| A�� | �������صĻ�ѧʽΪKO2��ÿ����������4��K+��4��O2- | |
| B�� | ������ÿ��K+��Χ��8��O2-��ÿ��O2-��Χ��8��K+ | |
| C�� | ��������ÿ��K+���������K+��8�� | |
| D�� | �����У�0������-2��������Ŀ��Ϊ2��1 |
���� A���þ����м����Ӹ���=8��$\frac{1}{8}$+6��$\frac{1}{2}$=4�����������Ӹ���=1+12��$\frac{1}{4}$=4�����Լ����Ӻͳ��������Ӹ���֮��=4��4=1��1��
B������ͼ֪��������ÿ��K+��Χ��6��O2-��ÿ��O2-��Χ��6��K+��
C����������ÿ��K+���������K+����=3��8��$\frac{1}{2}$��
D��ÿ��O2-��һ����λ����ɣ�ÿ��Oԭ��ƽ����$\frac{1}{2}$������ɣ�����ƽ����ɽ��м��㣮
��� �⣺A���þ����м����Ӹ���=8��$\frac{1}{8}$+6��$\frac{1}{2}$=4�����������Ӹ���=1+12��$\frac{1}{4}$=4�����Լ����Ӻͳ��������Ӹ���֮��=4��4=1��1�����Գ������صĻ�ѧʽΪKO2��ÿ����������4��K+��4��O2-����A��ȷ��
B������ͼ֪��������ÿ��K+��Χ��6��O2-��ÿ��O2-��Χ��6��K+����B����
C����������ÿ��K+���������K+����=3��8��$\frac{1}{2}$=12����C����
D��������K+��O2-�����ֱ�Ϊ4��4������1����������8����ԭ�ӣ����ݵ���غ�-2��Oԭ����ĿΪ2������0����ԭ����ĿΪ8-2=6�����Ծ����У�0����ԭ����-2����ԭ�ӵ���Ŀ��Ϊ3��1����D����
��ѡA��
���� ���⿼�龧�����㣬���ؿ���ѧ���ռ����������������������þ������Ȼ��ƾ������ƣ����Ȼ��ƾ�������֪ʶǨ�Ƶķ������н���״�ѡ����D��

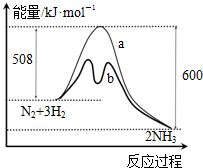
| A�� | �����¶ȿ���������Ӧ���ʣ������淴Ӧ���� | |
| B�� | ����Ӧ�Ļ�ܴ����淴Ӧ�Ļ�� | |
| C�� | a������δ�������ʱ�������仯���� | |
| D�� | �÷�Ӧ���Ȼ�ѧ����ʽΪ��2NH3?N2+3H2��H=-92 kJ•mol-1 |
| A�� | ���� | B�� | ���� | C�� | ������ | D�� | ������ |
| A�� | H2SO4 | B�� | MgCl2 | C�� | N2 | D�� | NH4Cl |
| A�� | ��ˮ | B�� | NaOH��Һ | C�� | NaCl��Һ | D�� | NH4Cl��Һ |
| A�� | ˮ��ɱ�������ͣ��ܶȱ�С | |
| B�� | ˮ���ȵ��ܸߵ��¶ȶ����Էֽ� | |
| C�� | HF��HCl��HBr��HI�����ȶ������μ��� | |
| D�� | CH4��SiH4��GeH4��SnH4�۵�����Է���������������� |
| A�� | ͭ���������Ƽ�����������Һ�к���ͭ���� | |
| B�� | �����������Ƽ�����������Һ�к���ͭ���� | |
| C�� | �����������Ƽ�����������Һ�к����������� | |
| D�� | ͭ���������Ƽ�����������Һ�к���п���� |
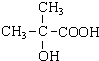 ��
��