��Ŀ����
ij���������̿�MnO2Լ70%��Al2 O3������п��ZnSԼ80%��FeS����ͬ����MnO2����Zn���ɵ��ԭ�ϣ����������£�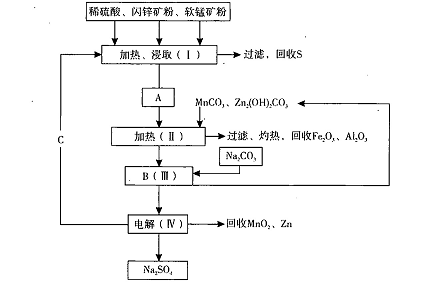
��֪����A��MnSO4��ZnSO4��Fe2(SO4)3��Al2(SO4)3�Ļ��Һ��
��IV�еĵ�ⷴӦʽΪMnSO4+ZnSO4+2H2O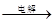 MnO2+Zn +2H2SO4��
MnO2+Zn +2H2SO4��
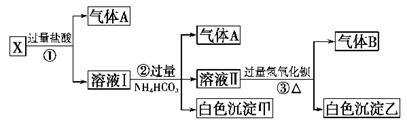
��1��A�����ڻ�ԭ������� ��
��2������MnCO3��Zn2(OH)2CO3�������� ��C�Ļ�ѧʽ�� ��
��3���������г��õ�Na2SO4��S�ȸ���Ʒ�⣬���ɵõ��ĸ���Ʒ�� ��
��4������ƷS�����������ᣬת�������ǣ�S��SO2��SO3��H2SO4��д���ڶ���ת���Ļ�ѧ����ʽ ��
��5��Ҫ��Na2SO4��Һ�еõ�â���� Na2SO4��10H2O��������еIJ���������Ũ���� ��
���ˡ�ϴ�ӡ�����ȡ�
��6��������MnO2��Zn�ĽǶȼ��㣬���̿����п��Ͷ�ϵ������ȴ�Լ�� ��
��1��MnSO4
(2)������Һ��p H,ʹFe3+��Al3+���ɳ��� H2SO4
��3��Fe2O3��Al2O3
(4) 2SO2+O2 2SO3
2SO3
(5) ��ȴ�ᾧ
��6��1��1(��1.03:1)
��������������Ƚ���Ϣ1��֪Mn���ϼ۽��ͣ����ڻ�ԭ�����ΪMnSO4���ɹ������̷�������MnCO3��Zn2(OH)2CO3��������������Һ��p H,ʹFe3+��Al3+���ɳ�������C�Ļ�ѧʽΪH2SO4�����ѵó��õ��ĸ���Ʒ����Fe2O3��Al2O3���������̿����п��������ֱ�Ϊx ��y�����ݢڿ�֪MnO2��Zn�����ʵ���֮��Ϊ1:1����0.7x g/87g/mol:0.8y g/97g/mol=1:1,���x/y=1.03:1.
���㣺������ԭ��Ӧ��Ԫ�ػ�����֪ʶ�����ʵķ�����ᴿ��

 ��У����ϵ�д�
��У����ϵ�д���������(ClO2)Ϊһ�ֻ���ɫ���壬�ǹ����Ϲ��ϵĸ�Ч�����ס����١���ȫ��ɱ����������
(1)��ҵ���Ʊ�ClO2�ķ�Ӧԭ��Ϊ2NaClO3��4HCl=2ClO2����Cl2����2H2O��2NaCl��
��Ũ�����ڷ�Ӧ����ʾ������������________��
| A��ֻ�л�ԭ�� | B����ԭ�Ժ����� |
| C��ֻ�������� | D�������Ժ����� |
(2)Ŀǰ�ѿ������õ�ⷨ��ȡClO2���¹��ա�
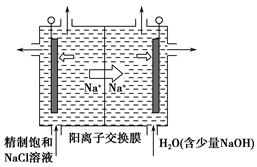
����ͼ����ʯī���缫��һ�������µ�ⱥ��ʳ��ˮ��ȡClO2��ʾ��ͼ������������ClO2�ĵ缫��ӦʽΪ_____________________________________��
�ڵ��һ��ʱ�䣬�������������������Ϊ112 mL(��״��)ʱ��ֹͣ��⡣ͨ�������ӽ���Ĥ�������ӵ����ʵ���Ϊ________mol����ƽ���ƶ�ԭ������������pH�����ԭ��______________________________________��
(3)ClO2����ˮ��Fe2����Mn2����S2����CN���������Ե�ȥ��Ч����ij������ˮ�к�CN��a mg/L������ClO2��CN���������������������������ɣ������ӷ�Ӧ����ʽΪ________________������100 m3������ˮ��������ҪClO2________mol��
ʳ���к���һ������þ���������ʣ��ӵ����е����ʧ��Ҫ���������ʡ�ˮ�֡������е������Լ����ա����ȶ�����ġ���֪��
�����ԣ�IO3-��Fe3����I2����ԭ�ԣ�S2O32-��I��
3I2��6OH��=5I����IO3-��3H2O
KI��I2 KI3
KI3
(1)ijѧϰС��Լӵ��ν���������ʵ�飺ȡһ����ij�ӵ���(���ܺ���KIO3��KI��Mg2����Fe3��)������������ˮ�ܽ⣬����ϡ�����ữ����������Һ��Ϊ3�ݡ���һ����Һ�еμ�KSCN��Һ���Ժ�ɫ���ڶ�����Һ�м�����KI���壬��Һ�Ե���ɫ����CCl4��ȡ���²���Һ���Ϻ�ɫ����������Һ�м�������KIO3����μӵ����Լ�����Һ����ɫ��
�ټ�KSCN��Һ�Ժ�ɫ���ú�ɫ������ (�û�ѧʽ��ʾ)��CCl4�����Ϻ�ɫ�������� (�õ���ʽ��ʾ)��
�ڵڶ�����Һ�м�������KI�����Ӧ�����ӷ���ʽΪ �� ��
(2)KI��Ϊ�ӵ����ʳ���ڱ�������У����ڿ��������������ã�������������ʧ��д����ʪ������KI��������Ӧ�Ļ�ѧ����ʽ�� ��
��I2����KI��Һ���ڵ��������£����Ƶ�KI3��H2O����������Ϊʳ�μӵ���Ƿ���ʣ� (��ǡ���)����˵�����ɣ� ��
(3)Ϊ����ӵ���(����KI)���ȶ��ԣ��ɼ��ȶ������ٵ����ʧ�������������п�����Ϊ�ȶ������� ��
| A��Na2S2O3 | B��AlCl3 |
| C��Na2CO3 | D��NaNO2 |