��Ŀ����
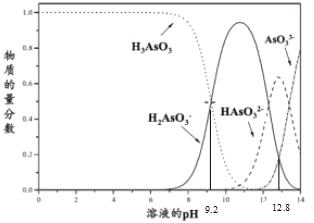
����Ŀ�����ֲ�ͬ�¶ȣ�T1��T2��ʱ�����ᱵ��ˮ�еij����ܽ�ƽ��������ͼ��ʾ��p(Ba2+)=-lgc(Ba2+)��p(SO42-)=-lgc(SO42-)����֪���ᱵ��ˮ���ܽ���������������˵����ȷ���ǣ� ��
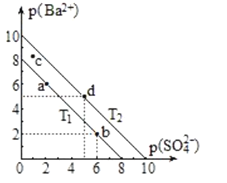
A.�¶ȸߵͣ�T1��T2
B.����BaCl2���壬��ʹ��Һ��a��䵽b��
C.c���Ӧ����Һ��T1�¶�ʱ�й�������
D.T2�¶��£�BaSO4��Ksp=1��10-25mol2��L-2
���𰸡�B
��������
A��BaSO4��ˮ���ܽ������������������¶ȴٽ�BaSO4�ܽ⣬���������¶�c(Ba2+)��ͬʱc(SO42-)������������ͬʱ������ԽС������ͼ֪���¶ȣ�T1��T2����A����
B������BaCl2���壬c(Ba2+)���������BaSO4�ܽ⣬ƽ������c(SO42-)��С����������������ͼ��B��ȷ��
C��T1�¶�c��Ϊ��������Һ��û�й�����������C����
D��T2�¶��£�p(Ba2+)=-lgc(Ba2+)=0ʱp(SO42-)=-lgc(SO42-)=10����c(Ba2+)=1mol/L��c(SO42-)=10-10 mol/L��Ksp=c(Ba2+)��c(SO42-)=1mol/L��10-10 mol/L=1��10-10mol2L-2����D����
��ѡ��B��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ��30��ʱ��������ͼװ�ý���ʵ�飬�����¼���±���ʾ��

ʵ�� | a�缫 | b�缫 | �������Һ | ���� |
I | Cu | Zn | ϡH2SO4 | ������ָ������ƫת |
II | Fe | Al | ϡH2SO4 | ������ָ������ƫת |
III | Fe | Al | ŨH2SO4 | ������ָ��������ƫת�������㣬a�缫������ڣ�b�缫������� |
IV | Fe | Al | ŨHNO3 | ������ָ��Ѹ������ƫת��a�缫���ܽ⣬b�缫������� |
����˵������ȷ����( )
A. II��AlΪ��������缫��Ӧ�ǣ�Al - 3e- �� Al3+
B. III�е�����˵��Fe��Al�����γ����ܵ�����Ĥ����ֹ�˵缫��Ӧ�Ľ���
C. IV��FeΪ������������������Ӧ
D. ����ʵ���������ͬ�����£�Fe��ŨHNO3�и��ȶ���Al��ŨH2SO4�и��ȶ�