��Ŀ����
18����һƿ�������Һ�����п��ܺ���NH${\;}_{4}^{+}$��K+��Mg2+��Ba2+��Al3+��Fe3+��SO${\;}_{4}^{2-}$��CO${\;}_{3}^{2-}$��NO${\;}_{3}^{-}$��I-��Cl-��ȡ����Һ��������ʵ�飺����pH��ֽ��ø���Һ�����ԣ�
��ȡ������Һ�������������Ƶ���ˮ������CCl4���������ú�CCl4����Ϻ�ɫ��
����ȡ������Һ����μ���ϡNaOH��Һ��ʹ��Һ��������Ϊ���ԣ��������μӹ������������ɣ�
��ȡ��������������Һ������Na2CO3��Һ���а�ɫ�������ɣ�
�ݽ��۵õ��ļ�����Һ���ȣ�������ų�����������ʹʪ��ĺ�ɫʯ����ֽ������
��������ʵ����ʵ�ش����⣺
��1��д������������Ӧ�����ӷ���ʽCl2+2I-�TI2+2Cl-��
��2������Һ�п϶����ڵ�������Ba2+��I-��NH4+��
��3��ȷ��NO${\;}_{3}^{-}$�Ƿ���ڵ����ɣ������������ӷ��̱�ʾ����8H++6I-+2NO3-�T2NO��+3I2+4H2O��
��4������Һ�л�����ȷ���Ƿ���ڵ�������K+��Cl-��
���� ���ݳ�����Һ�ã�ԭ��Һû�����Ӧ�����ӣ�
�ٸ���ʵ�飨1����Һ�������ж�������һ�����ڣ��������ӷ�Ӧ�����Ӳ��ܹ����棻
�ڸ���ʵ�飨2����������CCl4������������ˮ������CCl4����Ϻ�ɫ��˵����Һ��һ�����е����ӣ��ܹ�������ӷ�Ӧ�����Ӳ����棻
�۸���ʵ�飨3������NaOH��Һ��ʹ��Һ��������Ϊ���ԣ��ڵμӹ����к͵μ���Ϻ���Һ���������жϷ��������������ӷ�Ӧ���ɳ��������Ӳ����ڣ�
�ܸ���ʵ�飨4����ȡ��������������Һ��Na2CO3��Һ���а�ɫ�������ɣ���֪һ������̼������ӣ��ų�������ӷ�Ӧ�����ӣ�
������ɫ��Ӧ���麬�еĽ��������ӣ�
��� ����𡿽⣺�ٸ���ʵ�飨1��������Һ��ǿ���ԣ�˵����Һ�п϶�����H+����H+��CO32-��Ӧ������Ӧ�����ܹ��棬˵����Һ�п϶�������CO32-��
�ڸ���ʵ�飨2������CCl4����Ϻ�ɫ��˵����I2����������I-�����������������ģ��Ӷ�˵����Һ�к���I-����I-��Fe3+��NO3-��H+�ܷ���������ԭ��Ӧ�������ܹ��棬˵����Һ�п϶�������Fe3+��NO3-��
�۸���ʵ�飨3��������Һ��������Ϊ���ԣ��ڵμӹ����к͵μ���Ϻ���Һ��������������Fe3+��Mg2+��Al3+����Ӧ����������˵����Һ�п϶�������Fe3+��Mg2+��Al3+��
�ܸ���ʵ�飨4������ȡ����������������Һ��Na2CO3��Һ���а�ɫ�������ɣ�˵����Һ�п϶�����Ba2+����Ba2+����SO42-����������˵����Һ�в���SO42-��
��������ʵ����ʵȷ��������Һ�п϶����ڵ������ǣ�Ba2+��I-��NH4+��
�϶������ڵ������ǣ�Mg2+��Fe3+��Al3+��NO3-��Fe3+��CO32-��SO42-��
������ȷ���Ƿ���ڵ������ǣ�K+��Cl-��
��1������������Ӧ�����ӷ���ʽΪ��Cl2+2I-�TI2+2Cl-���ʴ�Ϊ��Cl2+2I-�TI2+2Cl-��
��2��������ʵ����ʵȷ��������Һ�п϶����ڵ������ǣ�Ba2+��I-��NH4+��
�ʴ�Ϊ��Ba2+��I-��NH4+��
��3����������Ӿ��������ԣ����Խ���ԭ�Եĵ���������Ϊ�ⵥ�ʣ��ⵥ���������۱��������Լ��飬��8H++6I-+2NO3-�T2NO��+3I2+4H2O���ʴ�Ϊ��8H++6I-+2NO3-�T2NO��+3I2+4H2O��
��4��������ȷ���Ƿ���ڵ������ǣ�K+��Cl-���ʴ�Ϊ��K+��Cl-��
���� ���⿼�����ʵļ��鼰����Ϊ��Ƶ���㣬���ճ�������֮��ķ�Ӧ�����Ӽ����Ϊ�ƶϵĹؼ������ط������ƶ��������ۺϿ��飬��Ŀ�ѶȲ���

 ϰ�⾫ѡϵ�д�
ϰ�⾫ѡϵ�д�| A�� | ��Ȼʯī�нϺõĵ����ԣ����������ڵ���� | |
| B�� | CaCO3��BaSO4��������ˮ�������Ƕ��ǵ���� | |
| C�� | �ƾ����������ˮ��Һ������״̬�¾����ܵ��磬���Ծƾ����ڷǵ���� | |
| D�� | ʵ��ⶨҺ̬HCl������KNO3�����ܵ��磬����HCl��KNO3���Ƿǵ���� |
���� ��� | �� | �� | �� |
| A | Cl2 | MgBr2 | NaOH |
| B | SO2 | Ca��OH��2 | NaHCO3 |
| C | SiO2 | NaOH | HF |
| D | NH3 | O2 | HNO3 |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | SO42-��NO3- | B�� | NO3-��Cl- | C�� | SO32-��NO3- | D�� | Cl-��NO3-��Na+ |
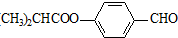 G��һ�ֺϳ�·�����£�
G��һ�ֺϳ�·�����£�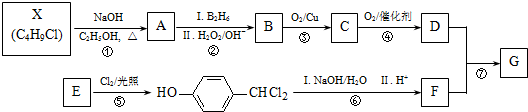
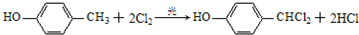 ��
�� ��
�� ��
�� ��1��ʹCl2��H2O��g��ͨ�����ȵ�̿�㣬����HCl��CO2���Ƿ��ȷ�Ӧ����1mol Cl2���뷴Ӧʱ�ͷ�145kJ��������д�������Ӧ���Ȼ�ѧ����ʽ��2Cl2��g��+2H2O��g��+C��s���T4HCl��g��+CO2��g����H=-290kJ•mol-1��
��1��ʹCl2��H2O��g��ͨ�����ȵ�̿�㣬����HCl��CO2���Ƿ��ȷ�Ӧ����1mol Cl2���뷴Ӧʱ�ͷ�145kJ��������д�������Ӧ���Ȼ�ѧ����ʽ��2Cl2��g��+2H2O��g��+C��s���T4HCl��g��+CO2��g����H=-290kJ•mol-1��
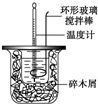
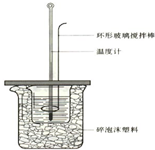 50mL 1.0mol•L-1�����50mL 1.1mol•L-1����������Һ����ͼװ���н����кͷ�Ӧ����ͨ���ⶨ��Ӧ���������ų��������������к��ȣ��Իش��������⣺
50mL 1.0mol•L-1�����50mL 1.1mol•L-1����������Һ����ͼװ���н����кͷ�Ӧ����ͨ���ⶨ��Ӧ���������ų��������������к��ȣ��Իش��������⣺