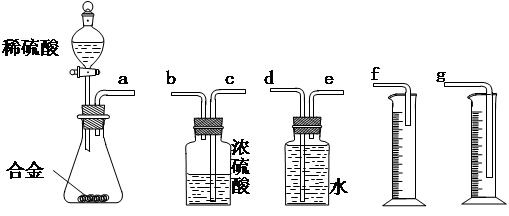
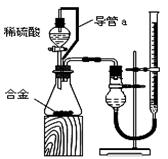
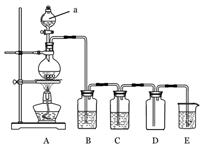
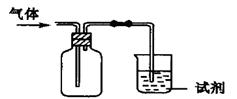
��Ŀ����
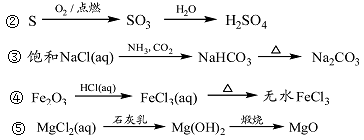
��12�֣�������NaOH��������100mL 0.5mol/L��NaOH��Һ�����ƹ�������ͼ��ʾ��
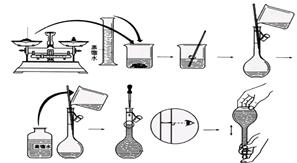
���������ش�
��1�����Ƹ���ҺӦѡ�� ������������
��2����______������ֽ��С�ձ�����������ƽ�ϳ�ȡ_____ NaOH���塣
��3��������ƿ�м�������ˮ����������˿̶��ߣ�Ӧ ��
��4�����ƺõ���Һ ����ܡ����ܡ������ڴ��������ƿ�С�
��5���������ػ����ʵ����ƫ�͵���

���������ش�
��1�����Ƹ���ҺӦѡ�� ������������
��2����______������ֽ��С�ձ�����������ƽ�ϳ�ȡ_____ NaOH���塣
��3��������ƿ�м�������ˮ����������˿̶��ߣ�Ӧ ��
��4�����ƺõ���Һ ����ܡ����ܡ������ڴ��������ƿ�С�
��5���������ػ����ʵ����ƫ�͵���
| A������ƿ��ԭ������������ˮ | B������ʱ�۲�Һ�温�� |
| C������ϴ�� | D������ʱ�۲�Һ������ |
��1��100 mL����ƿ��ֻ�� ����ƿ��һ�֣�
��2��С�ձ���2g(ֻ������0��)
��3������ ��4������ ��5��CD����һ����1�֣���һ����0�֣�
��2��С�ձ���2g(ֻ������0��)
��3������ ��4������ ��5��CD����һ����1�֣���һ����0�֣�
����һ�����ʵ���Ũ����Һ�����ơ�
��1��ȷ����100mL 0.5mol/L��NaOH��Һ��Ӧ��ѡ��100ml����ƿ��
��2��100mL 0.5mol/L��NaOH��Һ�к�������������0.1L��0.5mol/L��0.05mol��������m��0.05mol��40g/mol��2.0g������Ϊ�������ƾ�����ˮ�Ժ�ʴ�ԣ���˳���ʱӦ�÷���С�ձ��С�
��3��������ƿ�м�������ˮ����������˿̶��ߣ���ֻ�����������ơ�
��4������ƿ���ܳ�ʱ��ʢ����Һ�����������֮���뼰ʱת�Ƶ���Ӧ���Լ�ƿ�С�
��5������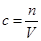 ��֪������ƿ��ԭ������������ˮ���Խ������Ӱ��ġ�����ʱ�۲�Һ�温�ӣ�������ƿ����Һ�����ƫ�٣�Ũ��ƫ����ѡ��D��ƫ�͡�����ϴ�ӣ������ʵ����ʵ���ƫ�٣�Ũ��ƫ�ͣ���ѡCD��
��֪������ƿ��ԭ������������ˮ���Խ������Ӱ��ġ�����ʱ�۲�Һ�温�ӣ�������ƿ����Һ�����ƫ�٣�Ũ��ƫ����ѡ��D��ƫ�͡�����ϴ�ӣ������ʵ����ʵ���ƫ�٣�Ũ��ƫ�ͣ���ѡCD��
��1��ȷ����100mL 0.5mol/L��NaOH��Һ��Ӧ��ѡ��100ml����ƿ��
��2��100mL 0.5mol/L��NaOH��Һ�к�������������0.1L��0.5mol/L��0.05mol��������m��0.05mol��40g/mol��2.0g������Ϊ�������ƾ�����ˮ�Ժ�ʴ�ԣ���˳���ʱӦ�÷���С�ձ��С�
��3��������ƿ�м�������ˮ����������˿̶��ߣ���ֻ�����������ơ�
��4������ƿ���ܳ�ʱ��ʢ����Һ�����������֮���뼰ʱת�Ƶ���Ӧ���Լ�ƿ�С�
��5������
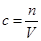 ��֪������ƿ��ԭ������������ˮ���Խ������Ӱ��ġ�����ʱ�۲�Һ�温�ӣ�������ƿ����Һ�����ƫ�٣�Ũ��ƫ����ѡ��D��ƫ�͡�����ϴ�ӣ������ʵ����ʵ���ƫ�٣�Ũ��ƫ�ͣ���ѡCD��
��֪������ƿ��ԭ������������ˮ���Խ������Ӱ��ġ�����ʱ�۲�Һ�温�ӣ�������ƿ����Һ�����ƫ�٣�Ũ��ƫ����ѡ��D��ƫ�͡�����ϴ�ӣ������ʵ����ʵ���ƫ�٣�Ũ��ƫ�ͣ���ѡCD��
��ϰ��ϵ�д�
�����Ŀ
 [����һ]
[����һ] 105Pa���������
105Pa���������