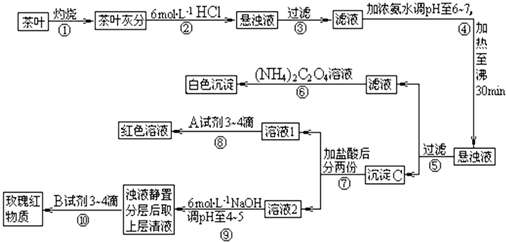
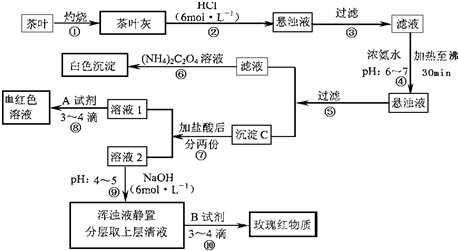
��Ŀ����
��14�֣������ҹ�����ϲ������Ʒ��ijУ��ѧ��ȤС���ͬѧ�������ʵ�������Լ����Ҷ�к��иơ����������ֽ���Ԫ�ء�
[���IJ�������]�������[(NH4)2C2O4]����������ʣ�����ƣ�CaC2O4��������ˮ��Ca2+��A13+��Fe3+��ȫ������pH��Ca(OH)2��pH��13��A1(OH)3��pH��5.5��Fe(OH)3��pH��4.1��
�Ը����������̼���Ϣ��գ�
��1������ڼ������������ _________________________________________ ��
��2��д������Ca2+�����ӷ���ʽ ___________________________��
��3��д������C������Ҫ���ʵĻ�ѧʽ _____________________________��
��4��д���������A�Լ����ɺ�ɫ��Һ�����ӷ���ʽ_____________________________��
��5�������������� ��
��6���²ⲽ����Ŀ���� _________________________________ ��
��7����֪��2Fe (s)+ 3/2O2 (g)= Fe2O3(s)����H = �� Q1 kJ��mol��1
2Al(s) + 3/2O2 (g)= Al 2O3(s)����H = �� Q2 kJ��mol��1
��Q1 ___________Q2�����������������=���� 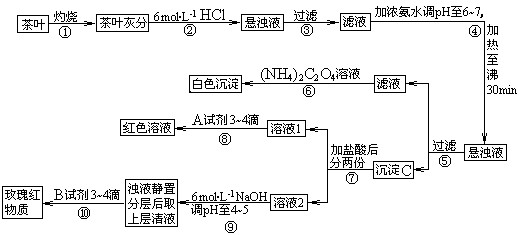
��1��ʹ��Ҷ�ҷ��еĸơ������������ܻ�����ת���ɿ������Ȼ��ʹCa2+��Al3+��Fe3+��������ʹCa2+��Al3+��Fe3+�ܽ⣩
��2��Ca2++(NH4)2C2O4=CaC2O4��+2NH4+ ��3��Fe(OH)3��Al(OH)3
��4��Fe3++3SCN Fe(SCN)3 ��5��ʹFe3+ת��ΪFe(OH)3������ʹ֮����Ԫ�ط���
Fe(SCN)3 ��5��ʹFe3+ת��ΪFe(OH)3������ʹ֮����Ԫ�ط���
��6������Al3+������Ԫ�أ����� ��7����
����