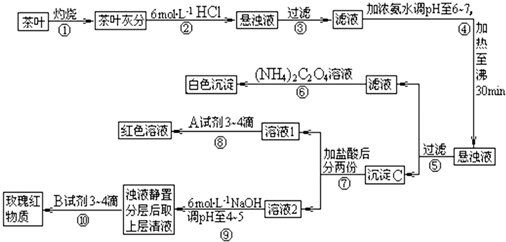
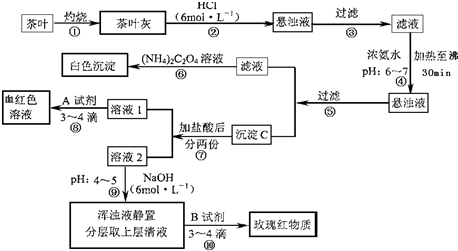
��Ŀ����
�����ҹ�����ϲ������Ʒ��ijУ��ѧ��ȤС���ͬѧ�������ʵ�������Լ����Ҷ�к���Ca��Al��Fe����Ԫ�ء������IJ������ϡ�����李�(NH4)2C2O4������������ʡ������(CaC2O4)������ˮ��Ca2+��Al3+��Fe3+��ȫ������pH��Ca(OH)2��pH��13��Al(OH)3��pH��5.5��Fe(OH)3��pH��4.1��

�Ը����������̼���Ϣ��գ�
(1)����ڼ������������____________________________________________________��
(2)д������Ca2+�����ӷ���ʽ________________________________________________��
(3)д������C������Ҫ���ʵĻ�ѧʽ____________��
(4)д���������A�Լ����ɺ�ɫ��Һ�����ӷ���ʽ______________________________��
(5)������������_________________���²ⲽ����Ŀ����______________________��
(1)ʹCa2+��Al3+��Fe3+����(��ʹCa2+��Al3+��Fe3+�ܽ�)
(2)Ca2++(NH4)![]() CaC2O4��+2
CaC2O4��+2![]()
(3)Fe(OH)3��Al(OH)3
(4)Fe3++3SCN-![]() Fe(SCN)3(д��������ȷ�����������ʽҲ��)
Fe(SCN)3(д��������ȷ�����������ʽҲ��)
(5)ʹFe3+ת��ΪFe(OH)3���� ����Al3+(����Ԫ��)����
������(1)���Ҷ�ҷ��м�����������ǽ����е�Ca2+��Al3+��Fe3+�ܽ⡣(2)�������pHΪ6��7ʱ����30 min����ʹAl3+��Fe3+ת����Al(OH)3��Fe(OH)3���������Ԣݹ��˺�������Һ�к�Ca2+������Ca2+�����ӷ���ʽΪCa2++(NH4)![]() CaC2O4��+2
CaC2O4��+2![]() ��(4)������Ǽ���Fe3+��
��(4)������Ǽ���Fe3+��