��Ŀ����
����������ȷ����
��һ��ƽ�й������䵰������Һʱ���Ӳ���ɿ���������ͨ·
��һ���¶Ⱥ�ѹǿ�£����������Ҫ������ӵ����ʵ�������
������Ħ�������ָ��λ���ʵ���������ռ��������䵥λ��L
����ͬ��ͬ���ʱ����������ʵ���Խ����ѹǿԽ��
����������ʱ��Ӧʹ������е�ˮ����ȫ���ɺ���ֹͣ����
�ޱ�״���£���lg��ƬͶ��20mL 18mol/L�������У���Ƭ��ȫ�ܽ�
| A���٢ڢ� | B���٢ۢ� | C���ڢۢ� | D���ڢܢ� |
A
�����������������������ˮ�����γɽ��壬����һ��ƽ�й������䵰������Һʱ���Ӳ���ɿ���������ͨ·������ȷ������������Ӽ�ľ���Զ�����������ֱ��������һ���¶Ⱥ�ѹǿ�£���ͬ������Ӽ�ľ��뼸��������ͬ�ģ��������������Ҫ������ӵ����ʵ�������������ȷ������Ħ�������ָ��λ���ʵ���������ռ��������䵥λ��L/mol���۲���ȷ������PV��nRT��֪����ͬ��ͬ���ʱ����������ʵ���Խ����ѹǿԽ����ȷ����������ʱ�����������г��ִ�������ʱ������ֹͣ���ȣ��������Ƚ�ʣ��ˮ�����ɣ��ݲ���ȷ���ڱ�״���½�������Ũ�����жۻ������������ܽ⣬����ȷ����ѡA��
���㣺���齺�����ʡ�Ӱ������������ء������ӵ����ɵ�Ӧ�á�����Ħ����������������Լ��ۻ���

 ��У����ϵ�д�
��У����ϵ�д���NA��ʾ�����ӵ�������ֵ������˵����ȷ����
| A����״���£�5.6 Lһ��������5.6 L������Ϻ�ķ�������Ϊ0.5NA |
| B��1 mol������Ӻ���8NA�����ۼ� |
| C��58.5 g���Ȼ��ƹ����к���NA���Ȼ��Ʒ��� |
| D����0.1 mol�������ƹ����У���������������0.1 NA |
��NA��ʾ�����ӵ�������ֵ������������ȷ����
| A����״���£�11.2LH2O���еķ�����Ϊ0.5NA |
| B������NA����ԭ�ӵĺ����ڱ�״���µ����ԼΪl1. 2L |
| C��25�棬l������ѹ�£�64gSO2�к��е�ԭ����Ϊ3NA |
| D���ڳ��³�ѹ�£�11. 2LCl2���еķ�����Ϊ0.5NA |
ijϡ�����ϡ����Ļ����Һ200 mL��ƽ���ֳ����ݡ�������һ��������ͭ�ۣ�������ܽ�19.2 g������һ�����������ۣ�������������������������ӵı仯����ͼ��ʾ�����з��������������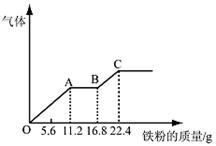
| A��ԭ�������NO3�����ʵ���Ũ��Ϊ2 mol/L |
| B��OA�β�������NO��AB�εķ�ӦΪFe+2Fe3+��3Fe2+��BC�β������� |
| C���ڶ�����Һ����������ΪFeSO4 |
| D��H2SO4Ũ��Ϊ2.5 mol��L��1 |
����˵���У���ȷ����( )��
| A��22��4 L N2�������ӵ������������� |
| B���ڱ�״���£�22��4 Lˮ������ԼΪ18 g |
| C��22 g������̼���״����11��2 L HCl������ͬ�ķ����� |
| D����״���£���ͬ������κ����嵥��������ԭ������ͬ |