��Ŀ����
ijϡ�����ϡ����Ļ����Һ200 mL��ƽ���ֳ����ݡ�������һ��������ͭ�ۣ�������ܽ�19.2 g������һ�����������ۣ�������������������������ӵı仯����ͼ��ʾ�����з��������������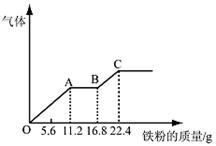
| A��ԭ�������NO3�����ʵ���Ũ��Ϊ2 mol/L |
| B��OA�β�������NO��AB�εķ�ӦΪFe+2Fe3+��3Fe2+��BC�β������� |
| C���ڶ�����Һ����������ΪFeSO4 |
| D��H2SO4Ũ��Ϊ2.5 mol��L��1 |
D
�������������A������ͼ���֪��������������OA�η�����ӦΪ��Fe+NO3-+4H+��Fe3++NO��+2H2O������ȫ�������������ã�����ԭ�������n��NO3-����n(Fe)�� ��0.2mol�����������Ũ�ȣ�0.2mol��0.1L��2.0mol/L����A��ȷ��B����ͼ���֪��������������OA�η�����ӦΪ��Fe+NO3-+4H+=Fe3++NO��+2H2O��AB�η�����ӦΪ��Fe+2Fe3+��3Fe2+����BC�η�����ӦΪ��Fe+2H+��Fe2++H2������B��ȷ��C������ȫ������ԭ��û�������Ե����ᣬ��Ϊ��Һ���������������������ȫ��ת��Ϊ�������ӣ�������Һ����������ΪFeSO4����C��ȷ��D����Ӧ��������22.4g�������ʵ�����22.4g��56g/mol��0.4mol�����е����������������У�����������غ��֪��ÿ�ݺ�����0.4mol�����������Ũ����0.4mol��0.1L��4mol/l����D����ѡD��
��0.2mol�����������Ũ�ȣ�0.2mol��0.1L��2.0mol/L����A��ȷ��B����ͼ���֪��������������OA�η�����ӦΪ��Fe+NO3-+4H+=Fe3++NO��+2H2O��AB�η�����ӦΪ��Fe+2Fe3+��3Fe2+����BC�η�����ӦΪ��Fe+2H+��Fe2++H2������B��ȷ��C������ȫ������ԭ��û�������Ե����ᣬ��Ϊ��Һ���������������������ȫ��ת��Ϊ�������ӣ�������Һ����������ΪFeSO4����C��ȷ��D����Ӧ��������22.4g�������ʵ�����22.4g��56g/mol��0.4mol�����е����������������У�����������غ��֪��ÿ�ݺ�����0.4mol�����������Ũ����0.4mol��0.1L��4mol/l����D����ѡD��
���㣺��������������ᡢ���ᷴӦ���йؼ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�ijʵ������������������һ�ֻ����Һ����֪��Һ��c��K+����c��Cl-���� c��Na+����c��SO42-����c��ʾ���ʵ���Ũ�ȣ����������ʿ�����
c��Na+����c��SO42-����c��ʾ���ʵ���Ũ�ȣ����������ʿ�����
| A��KCl��Na2SO4 | B��KCl��Na2SO4��NaCl |
| C��NaCl��Na2SO4��K2SO4 | D��KCl��K2SO4��Na2SO4 |
���б仯һ�����ڻ�ѧ�仯����
�ٷ绯 �ڱ�ɫ ��ȼ�� �ܱ�ը �ݰ���ת��ɺ��� ��ҵ������ �߾�����ˮ�����ɫ ����� ��NO2������ȴ����ɫ��dz �⳱��
| A���٢ڢۢܢ� | B���ۢܢݢ� | C���٢ۢܢߢ� | D���٢ۢݢߢ� |
����������ȷ����
��һ��ƽ�й������䵰������Һʱ���Ӳ���ɿ���������ͨ·
��һ���¶Ⱥ�ѹǿ�£����������Ҫ������ӵ����ʵ�������
������Ħ�������ָ��λ���ʵ���������ռ��������䵥λ��L
����ͬ��ͬ���ʱ����������ʵ���Խ����ѹǿԽ��
����������ʱ��Ӧʹ������е�ˮ����ȫ���ɺ���ֹͣ����
�ޱ�״���£���lg��ƬͶ��20mL 18mol/L�������У���Ƭ��ȫ�ܽ�
| A���٢ڢ� | B���٢ۢ� | C���ڢۢ� | D���ڢܢ� |
ij�����Ħ������ΪM g��mol��1��NA��ʾ�����ӵ�������ֵ����һ�����¶Ⱥ�ѹǿ�£����ΪVL�ĸ����������еķ�����ΪX����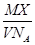 ��ʾ����
��ʾ����
| A��VL�����������(��gΪ��λ) |
| B��1L�����������(��gΪ��λ) |
| C��1mol����������(��LΪ��λ) |
| D��1L�������������ķ����� |
��Na2SO4��NaCl��NaOH�Ļ����Һ�У�Na+��SO��2-��OH-�ĸ�������8��1��2������Һ��Na2SO4��NaCl��NaOH�����ʵ���֮����
| A��1:1:1 | B��1:4:2 | C��1:2:4 | D��1:3:2 |
| | X | Y | Z |
| A | ������ | ������ | ������ |
| B | ���� | ��ɢϵ | ����� |
| C | ����� | �ᡢ��� | ������ |
| D | ���������� | ���������� | ������ |
���л�ѧ������ȷ����
A���ǻ��ĵ���ʽ��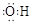 |
B��������10�����ӵ���ԭ�ӣ�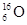 |
| C����ȩ�Ľṹ��ʽ��CH3COH |
D��̼ԭ�ӵĹ����ʾʽ��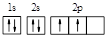 |
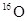 ��
�� ����Ԫ�ص����ֺ��أ�
����Ԫ�ص����ֺ��أ�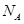 ��ʾ����٤������������˵����ȷ����
��ʾ����٤������������˵����ȷ����
A��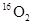 �� �� ��Ϊͬ���칹�� ��Ϊͬ���칹�� |
B��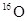 �� �� ��������Ų���ʽ��ͬ ��������Ų���ʽ��ͬ |
C��ͨ����ѧ�仯����ʵ��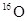 �� �� ����ת�� ����ת�� |
D����״���£�1.12L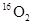 ��1.12L ��1.12L ������0.1 ������0.1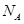 ����ԭ�� ����ԭ�� |
������������ȷ����
| A����28 gCO������ͬ��������C2H4������һ����28 g |
| B��1 molAl3�����еĺ��������Ϊ3NA |
| C�����³�ѹ�£�1 molL���麬�е�ԭ����Ϊ5NA |
| D��1 L 1 mol��L��1������Һ��CH3COO������ĿС��NA |